当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Association of intestinal anti-inflammatory drug target genes with psychiatric Disorders: A Mendelian randomization study
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-11 , DOI: 10.1016/j.jare.2024.05.002 Guorui Zhao , Zhe Lu , Yundan Liao , Yaoyao Sun , Yuyanan Zhang , Zhewei Kang , Xiaoyang Feng , Junyuan Sun , Weihua Yue
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-11 , DOI: 10.1016/j.jare.2024.05.002 Guorui Zhao , Zhe Lu , Yundan Liao , Yaoyao Sun , Yuyanan Zhang , Zhewei Kang , Xiaoyang Feng , Junyuan Sun , Weihua Yue
|
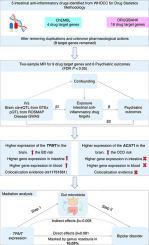
Psychiatric disorders present a substantial global public health burden with limited drug options. The gut-brain axis connects inflammatory bowel diseases and psychiatric disorders, which often have comorbidities. While some evidence hints at anti-inflammatory drugs aiding in treating psychiatric conditions, the specific effects of intestinal anti-inflammatory drugs remain unclear. This study investigates the causal effect of intestinal anti-inflammatory drug targets on psychiatric disorders. We hypothesize that these drug targets may offer new insights into the treatment and prevention of such disorders. Additionally, we explore gut microbiota’s mediating role between drug target genes and psychiatric disorders. We performed two-sample Mendelian randomization (MR) using summary data from existing expression quantitative trait loci (eQTL) and protein QTL in the brain, along with public genome-wide association studies of disease. We also explored gut microbiota’s mediating effect. The statistics encompassed six psychiatric disorders involving 9,725–500,199 individuals. Colocalization analysis enhanced the MR evidence. We uncovered a causal link between (a target of olsalazine) expression in the amygdala and bipolar disorder (BD) risk (odds ratio [OR] = 1.08; = 4.29 × 10). This association was observed even when the sigmoid colon and whole blood eQTL were considered as exposures. Colocalization analysis revealed a shared genetic variant (rs11751561) between expression and BD, with a posterior probability of 61.6 %. Interestingly, this causal effect was influenced by a decrease in the gut microbiota abundance of the genus (effect proportion = 10.05 %). Moreover, elevated expression was associated with higher obsessive–compulsive disorder risk (OR = 1.62; = 3.64 × 10; posterior probability = 3.1 %). These findings provide novel targets for the treatment of psychiatric disorders, underscore the potential of repurposing olsalazine, and emphasize the importance of and in future drug development.
中文翻译:

肠道抗炎药物靶基因与精神疾病的关联:孟德尔随机研究
精神疾病给全球公共卫生带来了巨大的负担,而药物选择却有限。肠-脑轴将炎症性肠病和精神疾病联系在一起,这些疾病通常有合并症。虽然一些证据暗示抗炎药物有助于治疗精神疾病,但肠道抗炎药物的具体作用仍不清楚。这项研究调查了肠道抗炎药物靶点对精神疾病的因果影响。我们假设这些药物靶点可能为此类疾病的治疗和预防提供新的见解。此外,我们还探讨了肠道微生物群在药物靶基因和精神疾病之间的中介作用。我们使用大脑中现有表达数量性状位点 (eQTL) 和蛋白质 QTL 的汇总数据以及公共疾病全基因组关联研究进行了两个样本孟德尔随机化 (MR)。我们还探讨了肠道微生物群的调节作用。统计数据涵盖六种精神疾病,涉及 9,725–500,199 人。共定位分析增强了 MR 证据。我们发现了杏仁核(奥沙拉嗪的靶标)表达与躁郁症 (BD) 风险之间的因果关系(比值比 [OR] = 1.08;= 4.29 × 10)。即使乙状结肠和全血 eQTL 被视为暴露,也观察到这种关联。共定位分析揭示了表达和 BD 之间共有的遗传变异 (rs11751561),后验概率为 61.6%。有趣的是,这种因果效应受到该属肠道微生物丰度下降的影响(效应比例 = 10.05%)。此外,表达升高与较高的强迫症风险相关(OR = 1.62;= 3.64 × 10;后验概率 = 3.1%)。这些发现为精神疾病的治疗提供了新的靶标,强调了奥沙拉嗪重新利用的潜力,并强调了未来药物开发的重要性。
更新日期:2024-05-11
中文翻译:

肠道抗炎药物靶基因与精神疾病的关联:孟德尔随机研究
精神疾病给全球公共卫生带来了巨大的负担,而药物选择却有限。肠-脑轴将炎症性肠病和精神疾病联系在一起,这些疾病通常有合并症。虽然一些证据暗示抗炎药物有助于治疗精神疾病,但肠道抗炎药物的具体作用仍不清楚。这项研究调查了肠道抗炎药物靶点对精神疾病的因果影响。我们假设这些药物靶点可能为此类疾病的治疗和预防提供新的见解。此外,我们还探讨了肠道微生物群在药物靶基因和精神疾病之间的中介作用。我们使用大脑中现有表达数量性状位点 (eQTL) 和蛋白质 QTL 的汇总数据以及公共疾病全基因组关联研究进行了两个样本孟德尔随机化 (MR)。我们还探讨了肠道微生物群的调节作用。统计数据涵盖六种精神疾病,涉及 9,725–500,199 人。共定位分析增强了 MR 证据。我们发现了杏仁核(奥沙拉嗪的靶标)表达与躁郁症 (BD) 风险之间的因果关系(比值比 [OR] = 1.08;= 4.29 × 10)。即使乙状结肠和全血 eQTL 被视为暴露,也观察到这种关联。共定位分析揭示了表达和 BD 之间共有的遗传变异 (rs11751561),后验概率为 61.6%。有趣的是,这种因果效应受到该属肠道微生物丰度下降的影响(效应比例 = 10.05%)。此外,表达升高与较高的强迫症风险相关(OR = 1.62;= 3.64 × 10;后验概率 = 3.1%)。这些发现为精神疾病的治疗提供了新的靶标,强调了奥沙拉嗪重新利用的潜力,并强调了未来药物开发的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号