当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anionic Conjugate Addition Oligomerization of Carbon Dioxide/Butadiene Derived Lactones
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-05-15 , DOI: 10.1021/acsmacrolett.4c00164 Brennan J. Crawford 1 , Calum Bochenek 2 , Luis D. Garcia Espinosa 1 , Addie R. Keating 2 , Kayla Williams-Pavlantos 1 , Chrys Wesdemiotis 1, 2 , James M. Eagan 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-05-15 , DOI: 10.1021/acsmacrolett.4c00164 Brennan J. Crawford 1 , Calum Bochenek 2 , Luis D. Garcia Espinosa 1 , Addie R. Keating 2 , Kayla Williams-Pavlantos 1 , Chrys Wesdemiotis 1, 2 , James M. Eagan 1
Affiliation
|
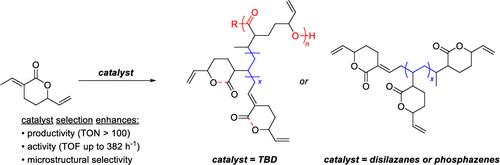
Nucleophilic and non-nucleophilic bases have been employed in anionic oligomerization of unsaturated δ-valerolactone (3-ethylidene-6-vinyltetrahydro-2H-pyran-2-one) (1). Compared to the seminal findings with 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), the unsaturated lactone reacts with guanidines, disilazanes, and phosphazenes both in bulk and in solution with higher productivities and activities, reaching full conversion with turnover frequencies up to 382 h–1. Additionally, reactions using phosphazenes and NaHMDS were active at 1 mol % catalyst loadings both in solvent and in bulk monomer at room temperature. Characterization of the reaction products by 1H, 13C, FTIR, MALDI-MS, tandem mass spectrometry (MS/MS), and ion mobility mass spectrometry (IM-MS) revealed microstructural differences dependent on the nucleophilicity of the organocatalytic base and reaction conditions. The products from phosphazene-catalyzed reactions are consistent with selective vinylogous 1,4-conjugate addition, whereas both conjugate addition and ring-opening mechanisms are observed in TBD. DSC reveals that these microstructures can be tuned to have a Tg range between −18 and 80 °C, while SEC and MALDI-MS reveal that only low molar mass oligomers are formed (748–5949 g/mol). From these results, an approach for selectively favoring the vinylogous 1,4-conjugate addition pathway is obtained over ring-opening reactivity.
中文翻译:

二氧化碳/丁二烯衍生内酯的阴离子共轭加成低聚
亲核和非亲核碱已用于不饱和 δ-戊内酯(3-亚乙基-6-乙烯基四氢-2H-吡喃-2-酮)的阴离子低聚 (1)。与 1,5,7-三氮杂双环[4.4.0]dec-5-ene (TBD) 的开创性发现相比,不饱和内酯与胍、二硅氮烷和磷腈在本体和溶液中反应具有更高的生产率和活性,达到完全转化,周转频率高达 382 小时 –1 。此外,在室温下,在溶剂和本体单体中,使用磷腈和 NaHMDS 的反应在 1 mol% 催化剂负载量下都很活跃。通过 1 H、 13 C、FTIR、MALDI-MS、串联质谱 (MS/MS) 和离子淌度质谱 (IM-MS) 对反应产物进行表征微观结构差异取决于有机催化碱的亲核性和反应条件。磷腈催化反应的产物与选择性插烯 1,4-共轭加成一致,而共轭加成和开环机制均在 TBD 中观察到。 DSC 显示这些微观结构可以调节至 -18 至 80 °C 之间的 T g 范围,而 SEC 和 MALDI-MS 显示仅形成低摩尔质量低聚物(748–5949 g/mol) )。从这些结果中,获得了一种选择性有利于插烯1,4-共轭加成途径而不是开环反应性的方法。
更新日期:2024-05-15
中文翻译:

二氧化碳/丁二烯衍生内酯的阴离子共轭加成低聚
亲核和非亲核碱已用于不饱和 δ-戊内酯(3-亚乙基-6-乙烯基四氢-2H-吡喃-2-酮)的阴离子低聚 (1)。与 1,5,7-三氮杂双环[4.4.0]dec-5-ene (TBD) 的开创性发现相比,不饱和内酯与胍、二硅氮烷和磷腈在本体和溶液中反应具有更高的生产率和活性,达到完全转化,周转频率高达 382 小时 –1 。此外,在室温下,在溶剂和本体单体中,使用磷腈和 NaHMDS 的反应在 1 mol% 催化剂负载量下都很活跃。通过 1 H、 13 C、FTIR、MALDI-MS、串联质谱 (MS/MS) 和离子淌度质谱 (IM-MS) 对反应产物进行表征微观结构差异取决于有机催化碱的亲核性和反应条件。磷腈催化反应的产物与选择性插烯 1,4-共轭加成一致,而共轭加成和开环机制均在 TBD 中观察到。 DSC 显示这些微观结构可以调节至 -18 至 80 °C 之间的 T g 范围,而 SEC 和 MALDI-MS 显示仅形成低摩尔质量低聚物(748–5949 g/mol) )。从这些结果中,获得了一种选择性有利于插烯1,4-共轭加成途径而不是开环反应性的方法。






































 京公网安备 11010802027423号
京公网安备 11010802027423号