当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Atomic Layer Etching of Gold Using Sulfuryl Chloride for Chlorination and Triethylphosphine for Ligand Addition
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-05-13 , DOI: 10.1021/acs.chemmater.4c00485
Jonathan L. Partridge 1 , Jessica A. Murdzek 1 , Virginia L. Johnson 1 , Andrew S. Cavanagh 1 , Sandeep Sharma 1 , Steven M. George 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-05-13 , DOI: 10.1021/acs.chemmater.4c00485
Jonathan L. Partridge 1 , Jessica A. Murdzek 1 , Virginia L. Johnson 1 , Andrew S. Cavanagh 1 , Sandeep Sharma 1 , Steven M. George 1
Affiliation
|
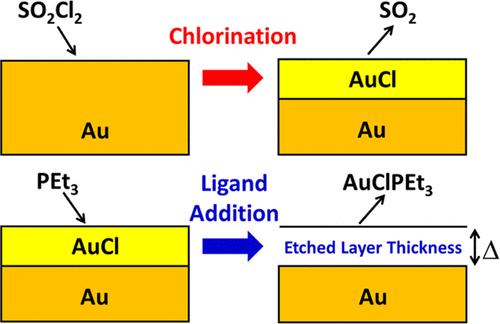
Thermal atomic layer etching (ALE) of gold was achieved using sequential chlorination and ligand-addition reactions. This two-step process first chlorinated gold using sulfuryl chloride (SO2Cl2) to form gold chloride. Subsequently, ligand addition to the gold chloride was performed using triethylphosphine (PEt3) to yield a volatile gold etch product. Quartz crystal microbalance measurements on cubic crystalline gold films showed etching at temperatures from 75 to 175 °C. The most consistent etch rate was 0.44 ± 0.16 Å/cycle at 150 °C. A mass increase was observed during each SO2Cl2 exposure when forming the gold chloride. A mass loss was then monitored during each PEt3 dose when ligand addition yielded a volatile etch product. In situ quadrupole mass spectrometry (QMS) studies on Au nanopowder at 150 °C showed the production of AuClPEt3 as the dominant Au-containing etch product during PEt3 exposures. Time-dependent QMS studies also observed the AuClPEt3+ ion intensity peaking at the beginning of each PEt3 exposure. The AuClPEt3+ ion intensity then decreased as the PEt3+ ion intensity remained constant. This behavior indicates a self-limiting ligand-addition reaction. X-ray photoelectron spectroscopy on these Au nanopowders showed evidence for AuClPEt3 on the surface of the Au nanopowder when the final exposure was PEt3. Transmission electron microscopy analysis revealed that Au ALE did not roughen the crystalline Au nanoparticles. Powder X-ray diffraction measurements also showed narrower diffraction peaks after Au ALE on Au nanoparticles that were consistent with larger Au nanoparticles. This sintering effect may be caused by Au redistribution resulting from the disproportionation of the AuCl surface species. Using the same alternating exposures of SO2Cl2 and PEt3, Cu and Ni nanopowders were also etched at 150 °C. Cu formed a volatile Cu2Cl2(PEt3)2 dimer during PEt3 exposures at 150 °C. Likewise, Ni formed volatile NiCl2(PEt3)2 during PEt3 exposures at 150 °C. Gibbs free energy changes from ab inito calculations support these etch product observations and offer a thermodynamic explanation for the formation of a copper dimer. These studies illustrate that sequential chlorination and ligand-addition reactions can provide a useful ALE pathway for gold and other metals.
中文翻译:

使用硫酰氯进行氯化和三乙基膦进行配体添加对金进行热原子层蚀刻
金的热原子层蚀刻(ALE)是通过连续氯化和配体加成反应实现的。该两步过程首先使用磺酰氯 (SO 2 Cl 2 ) 氯化金以形成氯化金。随后,使用三乙基膦(PEt 3 )将配体添加到氯化金中以产生挥发性金蚀刻产物。对立方晶体金薄膜的石英晶体微天平测量表明蚀刻温度为 75 至 175 °C。 150 °C 时最一致的蚀刻速率为 0.44 ± 0.16 Å/周期。当形成氯化金时,在每次 SO 2 Cl 2 暴露期间观察到质量增加。然后,当配体添加产生挥发性蚀刻产物时,在每次 PEt 3 剂量期间监测质量损失。在 150 °C 对 Au 纳米粉末进行的原位四极杆质谱 (QMS) 研究表明,在 PEt 3 暴露过程中,AuClPEt 3 是主要的含 Au 蚀刻产物。时间依赖性 QMS 研究还观察到 AuClPEt 3 + 离子强度在每次 PEt 3 暴露开始时达到峰值。 AuClPEt 3 + 离子强度随后降低,而 PEt 3 + 离子强度保持恒定。这种行为表明自限性配体加成反应。这些 Au 纳米粉末的 X 射线光电子能谱表明,当最终曝光为 PEt 3 时,Au 纳米粉末表面上存在 AuClPEt 3 。透射电子显微镜分析表明,Au ALE 不会使结晶金纳米颗粒变得粗糙。粉末 X 射线衍射测量还显示,在 Au ALE 处理 Au 纳米颗粒后,衍射峰变窄,这与较大的 Au 纳米颗粒一致。 这种烧结效应可能是由 AuCl 表面物质歧化导致的 Au 重新分布引起的。使用相同的 SO 2 Cl 2 和 PEt 3 交替暴露,Cu 和 Ni 纳米粉末也在 150 °C 下进行蚀刻。在 150 ℃暴露于 PEt 3 期间,Cu 形成挥发性 Cu 2 Cl 2 (PEt 3 ) 2 二聚体°C。同样,在 150 °C 暴露于 PEt 3 期间,Ni 形成挥发性 NiCl 2 (PEt 3 ) 2 。从头开始计算的吉布斯自由能变化支持了这些蚀刻产物的观察结果,并为铜二聚体的形成提供了热力学解释。这些研究表明,连续氯化和配体加成反应可以为金和其他金属提供有用的 ALE 途径。
更新日期:2024-05-13
中文翻译:

使用硫酰氯进行氯化和三乙基膦进行配体添加对金进行热原子层蚀刻
金的热原子层蚀刻(ALE)是通过连续氯化和配体加成反应实现的。该两步过程首先使用磺酰氯 (SO 2 Cl 2 ) 氯化金以形成氯化金。随后,使用三乙基膦(PEt 3 )将配体添加到氯化金中以产生挥发性金蚀刻产物。对立方晶体金薄膜的石英晶体微天平测量表明蚀刻温度为 75 至 175 °C。 150 °C 时最一致的蚀刻速率为 0.44 ± 0.16 Å/周期。当形成氯化金时,在每次 SO 2 Cl 2 暴露期间观察到质量增加。然后,当配体添加产生挥发性蚀刻产物时,在每次 PEt 3 剂量期间监测质量损失。在 150 °C 对 Au 纳米粉末进行的原位四极杆质谱 (QMS) 研究表明,在 PEt 3 暴露过程中,AuClPEt 3 是主要的含 Au 蚀刻产物。时间依赖性 QMS 研究还观察到 AuClPEt 3 + 离子强度在每次 PEt 3 暴露开始时达到峰值。 AuClPEt 3 + 离子强度随后降低,而 PEt 3 + 离子强度保持恒定。这种行为表明自限性配体加成反应。这些 Au 纳米粉末的 X 射线光电子能谱表明,当最终曝光为 PEt 3 时,Au 纳米粉末表面上存在 AuClPEt 3 。透射电子显微镜分析表明,Au ALE 不会使结晶金纳米颗粒变得粗糙。粉末 X 射线衍射测量还显示,在 Au ALE 处理 Au 纳米颗粒后,衍射峰变窄,这与较大的 Au 纳米颗粒一致。 这种烧结效应可能是由 AuCl 表面物质歧化导致的 Au 重新分布引起的。使用相同的 SO 2 Cl 2 和 PEt 3 交替暴露,Cu 和 Ni 纳米粉末也在 150 °C 下进行蚀刻。在 150 ℃暴露于 PEt 3 期间,Cu 形成挥发性 Cu 2 Cl 2 (PEt 3 ) 2 二聚体°C。同样,在 150 °C 暴露于 PEt 3 期间,Ni 形成挥发性 NiCl 2 (PEt 3 ) 2 。从头开始计算的吉布斯自由能变化支持了这些蚀刻产物的观察结果,并为铜二聚体的形成提供了热力学解释。这些研究表明,连续氯化和配体加成反应可以为金和其他金属提供有用的 ALE 途径。




































 京公网安备 11010802027423号
京公网安备 11010802027423号