Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu–Mo Dual Sites in Cu-Doped MoSe2 for Enhanced Electrosynthesis of Urea
ACS Nano ( IF 15.8 ) Pub Date : 2024-05-13 , DOI: 10.1021/acsnano.4c01821
Jiadi Jiang 1 , Guanzheng Wu 1 , Mengmiao Sun 1 , Yi Liu 1 , Yidong Yang 1 , Aijun Du 2 , Lei Dai 3 , Xin Mao 2 , Qing Qin 1
ACS Nano ( IF 15.8 ) Pub Date : 2024-05-13 , DOI: 10.1021/acsnano.4c01821
Jiadi Jiang 1 , Guanzheng Wu 1 , Mengmiao Sun 1 , Yi Liu 1 , Yidong Yang 1 , Aijun Du 2 , Lei Dai 3 , Xin Mao 2 , Qing Qin 1
Affiliation
|
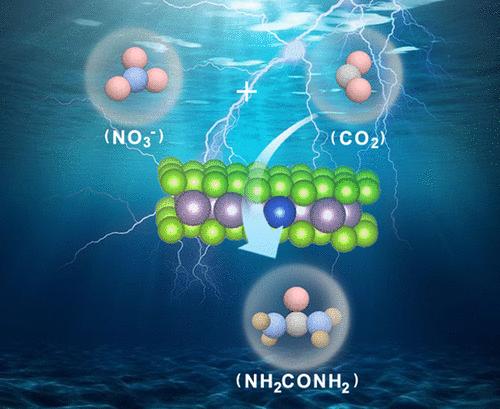
The quest for sustainable urea production has directed attention toward electrocatalytic methods that bypass the energy-intensive traditional Haber–Bosch process. This study introduces an approach to urea synthesis through the coreduction of CO2 and NO3– using copper-doped molybdenum diselenide (Cu–MoSe2) with Cu–Mo dual sites as electrocatalysts. The electrocatalytic activity of the Cu–MoSe2 electrode is characterized by a urea yield rate of 1235 μg h–1 mgcat.–1 at −0.7 V versus the reversible hydrogen electrode and a maximum Faradaic efficiency of 23.43% at −0.6 V versus RHE. Besides, a continuous urea production with an enhanced average yield rate of 9145 μg h–1 mgcat.–1 can be achieved in a flow cell. These figures represent a substantial advancement over that of the baseline MoSe2 electrode. Density functional theory (DFT) calculations elucidate that Cu doping accelerates *NO2 deoxygenation and significantly decreases the energy barriers for C–N bond formation. Consequently, Cu–MoSe2 demonstrates a more favorable pathway for urea production, enhancing both the efficiency and feasibility of the process. This study offers valuable insights into electrode design and understanding of the facilitated electrochemical pathways.
中文翻译:

Cu 掺杂 MoSe2 中的 Cu-Mo 双位点用于增强尿素电合成
对可持续尿素生产的追求已将注意力转向绕过能源密集型传统哈伯-博世工艺的电催化方法。本研究介绍了一种通过 CO 2和 NO 3共还原合成尿素的方法-使用具有 Cu-Mo 双位点的铜掺杂二硒化钼 (Cu-MoSe 2 ) 作为电催化剂。 Cu-MoSe 2电极的电催化活性特点是尿素产率为 1235 μg h –1 mg cat。与可逆氢电极相比,-0.7 V 时为–1 ,与 RHE 相比,-0.6 V 时最大法拉第效率为 23.43%。此外,连续尿素生产的平均产量提高到 9145 µg h –1 mg cat。 –1可以在流通池中实现。这些数字代表了相对于基线 MoSe 2电极的显着进步。密度泛函理论 (DFT) 计算表明,Cu 掺杂可加速*NO 2脱氧并显着降低 C-N 键形成的能垒。因此,Cu-MoSe 2展示了一种更有利的尿素生产途径,提高了工艺的效率和可行性。这项研究为电极设计和促进电化学途径的理解提供了宝贵的见解。
更新日期:2024-05-13
中文翻译:

Cu 掺杂 MoSe2 中的 Cu-Mo 双位点用于增强尿素电合成
对可持续尿素生产的追求已将注意力转向绕过能源密集型传统哈伯-博世工艺的电催化方法。本研究介绍了一种通过 CO 2和 NO 3共还原合成尿素的方法-使用具有 Cu-Mo 双位点的铜掺杂二硒化钼 (Cu-MoSe 2 ) 作为电催化剂。 Cu-MoSe 2电极的电催化活性特点是尿素产率为 1235 μg h –1 mg cat。与可逆氢电极相比,-0.7 V 时为–1 ,与 RHE 相比,-0.6 V 时最大法拉第效率为 23.43%。此外,连续尿素生产的平均产量提高到 9145 µg h –1 mg cat。 –1可以在流通池中实现。这些数字代表了相对于基线 MoSe 2电极的显着进步。密度泛函理论 (DFT) 计算表明,Cu 掺杂可加速*NO 2脱氧并显着降低 C-N 键形成的能垒。因此,Cu-MoSe 2展示了一种更有利的尿素生产途径,提高了工艺的效率和可行性。这项研究为电极设计和促进电化学途径的理解提供了宝贵的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号