Intensive Care Medicine ( IF 27.1 ) Pub Date : 2024-05-13 , DOI: 10.1007/s00134-024-07448-x Morgan T Rose 1, 2, 3, 4 , Natasha E Holmes 1, 4, 5 , Glenn M Eastwood 6 , Sara Vogrin 7 , Fiona James 1 , Joseph F De Luca 1, 4 , Rinaldo Bellomo 6, 8, 9, 10 , Stephen J Warrillow 6, 9 , Michelle Phung 11, 12 , Sara L Barnes 13 , Brendan Murfin 14 , Ben Rogers 15, 16 , Belinda Lambros 2, 3 , Brennan Collis 17 , Trisha N Peel 17, 18 , Monica A Slavin 2, 3, 19, 20 , Jason A Trubiano 1, 2, 4
|
Purpose
Critically ill patients are vulnerable to penicillin allergy labels that may be incorrect. The validity of skin testing in intensive care units (ICUs) is uncertain. Many penicillin allergy labels are low risk, and validated tools exist to identify those amenable to direct oral challenge. This pilot randomised controlled trial explored the feasibility, safety, and validity of direct enteral challenge for low-risk penicillin allergy labels in critical illness.
Methods
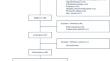
Consenting patients with a low-risk penicillin allergy label (PAL) (PEN-FAST risk assessment score < 3) in four ICUs (Melbourne, Australia) were randomised 1:1 to penicillin (250 mg amoxicillin or implicated penicillin) direct enteral challenge versus routine care (2-h post-randomisation observation for each arm). Repeat challenge was performed post -ICU in the intervention arm. Patients were reviewed at 24 h and 5 days after each challenge/observation.
Results
We screened 533 patients. 130 (24.4%) were eligible and 80/130 (61.5%) enrolled (age median 64.5 years (interquartile range, IQR 53.5, 74), PEN-FAST median 1 (IQR 0,1)), with 40 (50%) randomised to direct enteral challenge. A positive challenge rate of 2.5% was identified. No antibiotic-associated serious adverse events were identified. 32/40 (80%) received a repeat challenge (zero positive). Post-randomisation, 13 (32%) of the intervention arm and 4 (10%) of the control arm received penicillin (odds ratio, OR 4.33 [1.27, 14.78] p = 0.019).
Conclusion
These findings support the safety, validity, and feasibility of direct enteral challenge for critically ill patients with PEN-FAST assessed low-risk penicillin allergy. The absence of false negative results was confirmed by subsequent negative repeat challenges. A relatively low recruitment to screened ratio suggests that more inclusive eligibility criteria and integration of allergy assessment into routine ICU processes are needed to optimise allergy delabelling in critical illness.
中文翻译:

口服挑战与常规护理比较评估危重病医院患者的低风险青霉素过敏(ORACLE):一项试点安全性和可行性随机对照试验
目的
危重患者很容易受到可能不正确的青霉素过敏标签的影响。重症监护病房 (ICU) 中皮肤测试的有效性尚不确定。许多青霉素过敏标签风险较低,并且存在经过验证的工具来识别那些适合直接口服挑战的过敏标签。这项随机对照试验探讨了危重疾病中低风险青霉素过敏标签直接肠内激发的可行性、安全性和有效性。
方法
四个 ICU(澳大利亚墨尔本)同意的低风险青霉素过敏标签 (PAL)(PEN-FAST 风险评估评分 < 3)患者按 1:1 随机分配至青霉素(250 毫克阿莫西林或牵连青霉素)直接肠内激发组常规护理(每组随机化后观察 2 小时)。干预组在 ICU 后进行重复挑战。每次挑战/观察后 24 小时和 5 天对患者进行审查。
结果
我们筛查了 533 名患者。 130 名 (24.4%) 符合资格,80/130 (61.5%) 名入组(年龄中位数 64.5 岁(四分位数间距,IQR 53.5, 74),PEN-FAST 中位数 1 (IQR 0,1)),其中 40 名(50%)随机分配至直接肠内挑战。确定阳性挑战率为 2.5%。没有发现与抗生素相关的严重不良事件。 32/40 (80%) 接受了重复挑战(零阳性)。随机分组后,干预组中有 13 名 (32%) 患者和对照组有 4 名患者 (10%) 接受了青霉素治疗(比值比,OR 4.33 [1.27, 14.78] p = 0.019)。
结论
这些发现支持对经 PEN-FAST 评估的低风险青霉素过敏的危重患者进行直接肠内激发的安全性、有效性和可行性。随后的阴性重复挑战证实不存在假阴性结果。相对较低的招募与筛查比率表明,需要更具包容性的资格标准并将过敏评估纳入常规 ICU 流程,以优化危重疾病的过敏脱标签。











































 京公网安备 11010802027423号
京公网安备 11010802027423号