当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonalternant Nanographenes Containing N-Centered Cyclopenta[ef]heptalene and Aza[7]Helicene Units
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-05-08 , DOI: 10.1021/jacs.4c03815 Shuhai Qiu 1 , Abel Cárdenas Valdivia 2 , Weiwen Zhuang 1 , Faan-Fung Hung 1 , Chi-Ming Che 1, 3 , Juan Casado 2 , Junzhi Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-05-08 , DOI: 10.1021/jacs.4c03815 Shuhai Qiu 1 , Abel Cárdenas Valdivia 2 , Weiwen Zhuang 1 , Faan-Fung Hung 1 , Chi-Ming Che 1, 3 , Juan Casado 2 , Junzhi Liu 1
Affiliation
|
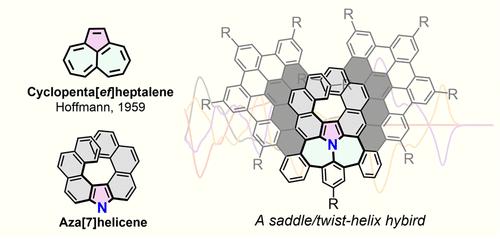
Introducing helical subunits into negatively curved π-systems has a significant effect on both the molecular geometry and photophysical properties; however, the synthesis of these helical π-systems embedded with nonbenzenoid subunits remains challenging due to the high strain deriving from both the curvature and helix. Here, we report a family of nonalternant nanographenes containing a nitrogen (N)-doped cyclopenta[ef]heptalene unit. Among them, CPH-2 and CPH-3 can be viewed as hybrids of benzoannulated cyclopenta[ef]heptalene and aza[7]helicene. The crystal structures revealed a saddle geometry for CPH-1, a saddle-helix hybrid for CPH-2, and a twist-helix hybrid for CPH-3. Experimental measurements and theoretical calculations indicate that the saddle moieties in CPHs undergo flexible conformational changes at room temperature, while the aza[7]helicene subunit exhibits a dramatically increased racemization energy barrier (78.2 kcal mol–1 for CPH-2, 143.2 kcal mol–1 for CPH-3). The combination of the nitrogen lone electron pairs of the N-doped cyclopenta[ef]heptalene unit with the twisted helix fragments results in rich photophysics with distinctive fluorescence and phosphorescence in CPH-1 and CPH-2 and the similar energy fluorescence and phosphorescence in CPH-3. Both enantiopure CPH-2 and CPH-3 display distinct circular dichroism (CD) signals in the UV–vis range. Notably, compared to the reported fully π-extended helical nanographenes, CPH-3 exhibits excellent chiroptical properties with a |gabs| value of 1.0 × 10–2 and a |glum| value of 7.0 × 10–3; these values are among the highest for helical nanographenes.
中文翻译:

含有N中心环戊二烯和氮杂[7]螺旋烯单元的非交替纳米石墨烯
将螺旋亚基引入负弯曲π-系统对分子几何形状和光物理性质具有显着影响;然而,由于曲率和螺旋产生的高应变,这些嵌入非苯环亚基的螺旋 π 系统的合成仍然具有挑战性。在这里,我们报道了一系列含有氮(N)掺杂的环戊二烯[ ef ]庚烯单元的非交替纳米石墨烯。其中, CPH-2和CPH-3可以看作是苯并环化的环戊[ ef ]庚烯和氮杂[7]螺烯的杂化物。晶体结构揭示了CPH-1的鞍形几何结构、 CPH-2的鞍形螺旋杂化结构以及CPH-3的扭转螺旋杂化结构。实验测量和理论计算表明, CPH中的鞍形部分在室温下经历灵活的构象变化,而氮杂[7]螺旋烯亚基表现出显着增加的外消旋能垒( CPH-2为 78.2 kcal mol –1,143.2 kcal mol – 1对于CPH-3 )。 N掺杂环戊二烯[ ef ]庚烯单元的氮孤电子对与扭曲螺旋片段的结合产生了丰富的光物理特性,在CPH-1和CPH-2中具有独特的荧光和磷光,在CPH中具有相似的能量荧光和磷光-3 。对映体纯CPH-2和CPH-3在紫外-可见光范围内显示出明显的圆二色性 (CD) 信号。 值得注意的是,与报道的完全π延伸螺旋纳米石墨烯相比, CPH-3表现出优异的手性光学特性, g腹肌|值 1.0 × 10 –2和 |克伦|值为 7.0 × 10 –3 ;这些值是螺旋纳米石墨烯中最高的。
更新日期:2024-05-08
中文翻译:

含有N中心环戊二烯和氮杂[7]螺旋烯单元的非交替纳米石墨烯
将螺旋亚基引入负弯曲π-系统对分子几何形状和光物理性质具有显着影响;然而,由于曲率和螺旋产生的高应变,这些嵌入非苯环亚基的螺旋 π 系统的合成仍然具有挑战性。在这里,我们报道了一系列含有氮(N)掺杂的环戊二烯[ ef ]庚烯单元的非交替纳米石墨烯。其中, CPH-2和CPH-3可以看作是苯并环化的环戊[ ef ]庚烯和氮杂[7]螺烯的杂化物。晶体结构揭示了CPH-1的鞍形几何结构、 CPH-2的鞍形螺旋杂化结构以及CPH-3的扭转螺旋杂化结构。实验测量和理论计算表明, CPH中的鞍形部分在室温下经历灵活的构象变化,而氮杂[7]螺旋烯亚基表现出显着增加的外消旋能垒( CPH-2为 78.2 kcal mol –1,143.2 kcal mol – 1对于CPH-3 )。 N掺杂环戊二烯[ ef ]庚烯单元的氮孤电子对与扭曲螺旋片段的结合产生了丰富的光物理特性,在CPH-1和CPH-2中具有独特的荧光和磷光,在CPH中具有相似的能量荧光和磷光-3 。对映体纯CPH-2和CPH-3在紫外-可见光范围内显示出明显的圆二色性 (CD) 信号。 值得注意的是,与报道的完全π延伸螺旋纳米石墨烯相比, CPH-3表现出优异的手性光学特性, g腹肌|值 1.0 × 10 –2和 |克伦|值为 7.0 × 10 –3 ;这些值是螺旋纳米石墨烯中最高的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号