Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An ATP13A1-assisted topogenesis pathway for folding multi-spanning membrane proteins
Molecular Cell ( IF 14.5 ) Pub Date : 2024-05-08 , DOI: 10.1016/j.molcel.2024.04.010
Jia Ji 1 , Meng-Ke Cui 1 , Rong Zou 1 , Ming-Zhi Wu 1 , Man-Xi Ge 1 , Jiqiang Li 1 , Zai-Rong Zhang 1
Molecular Cell ( IF 14.5 ) Pub Date : 2024-05-08 , DOI: 10.1016/j.molcel.2024.04.010
Jia Ji 1 , Meng-Ke Cui 1 , Rong Zou 1 , Ming-Zhi Wu 1 , Man-Xi Ge 1 , Jiqiang Li 1 , Zai-Rong Zhang 1
Affiliation
|
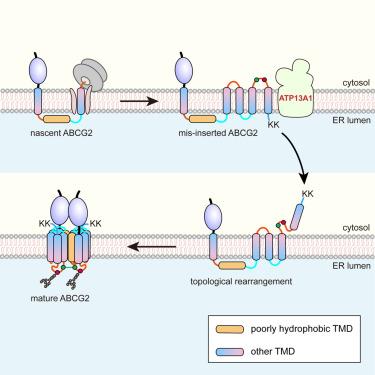
Many multi-spanning membrane proteins contain poorly hydrophobic transmembrane domains (pTMDs) protected from phospholipid in mature structure. Nascent pTMDs are difficult for translocon to recognize and insert. How pTMDs are discerned and packed into mature, muti-spanning configuration remains unclear. Here, we report that pTMD elicits a post-translational topogenesis pathway for its recognition and integration. Using six-spanning protein adenosine triphosphate-binding cassette transporter G2 (ABCG2) and cultured human cells as models, we show that ABCG2’s pTMD2 can pass through translocon into the endoplasmic reticulum (ER) lumen, yielding an intermediate with inserted yet mis-oriented downstream TMDs. After translation, the intermediate recruits P5A-ATPase ATP13A1, which facilitates TMD re-orientation, allowing further folding and the integration of the remaining lumen-exposed pTMD2. Depleting ATP13A1 or disrupting pTMD-characteristic residues arrests intermediates with mis-oriented and exposed TMDs. Our results explain how a “difficult” pTMD is co-translationally skipped for insertion and post-translationally buried into the final correct structure at the late folding stage to avoid excessive lipid exposure.
中文翻译:

一种用于折叠多跨膜蛋白的 ATP13A1 辅助拓扑发生通路
许多多跨膜蛋白在成熟结构中含有防磷脂保护的疏水性差的跨膜结构域 (pTMD)。新生的 pTMD 难以被 translocon 识别和插入。pTMD 如何被识别并打包成成熟的多跨构型仍不清楚。在这里,我们报道了 pTMD 引发翻译后拓扑发生途径以进行其识别和整合。使用六跨蛋白三磷酸腺苷结合盒转运蛋白 G2 (ABCG2) 和培养的人类细胞作为模型,我们表明 ABCG2 的 pTMD2 可以通过转位子进入内质网 (ER) 腔,产生带有插入但方向错误的下游 TMD 的中间体。翻译后,中间体募集 P5A-ATP 酶 ATP13A1,这促进了 TMD 的重新定位,允许进一步折叠和整合剩余的腔暴露的 pTMD2。耗尽 ATP13A1 或破坏 pTMD 特征残基会阻止具有错误定向和暴露的 TMD 的中间体。我们的结果解释了 “困难” pTMD 如何在折叠后期共翻译跳过插入并在翻译后埋入最终的正确结构中,以避免过度的脂质暴露。
更新日期:2024-05-08
中文翻译:

一种用于折叠多跨膜蛋白的 ATP13A1 辅助拓扑发生通路
许多多跨膜蛋白在成熟结构中含有防磷脂保护的疏水性差的跨膜结构域 (pTMD)。新生的 pTMD 难以被 translocon 识别和插入。pTMD 如何被识别并打包成成熟的多跨构型仍不清楚。在这里,我们报道了 pTMD 引发翻译后拓扑发生途径以进行其识别和整合。使用六跨蛋白三磷酸腺苷结合盒转运蛋白 G2 (ABCG2) 和培养的人类细胞作为模型,我们表明 ABCG2 的 pTMD2 可以通过转位子进入内质网 (ER) 腔,产生带有插入但方向错误的下游 TMD 的中间体。翻译后,中间体募集 P5A-ATP 酶 ATP13A1,这促进了 TMD 的重新定位,允许进一步折叠和整合剩余的腔暴露的 pTMD2。耗尽 ATP13A1 或破坏 pTMD 特征残基会阻止具有错误定向和暴露的 TMD 的中间体。我们的结果解释了 “困难” pTMD 如何在折叠后期共翻译跳过插入并在翻译后埋入最终的正确结构中,以避免过度的脂质暴露。

































 京公网安备 11010802027423号
京公网安备 11010802027423号