当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective hydrogenation of furfural to furfuryl alcohol over copper-cobalt bimetallic catalyst
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-05-03 , DOI: 10.1016/j.cej.2024.151677 Jingyun Zhang , Yumeng Liu , Zhen Jia , ShitaoYu , Shiwei Liu , Lu Li , Qiong Wu , Hailong Yu , Yuxiang Liu , Xiaoqing Jiang , Yue Liu , Chao Xu
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-05-03 , DOI: 10.1016/j.cej.2024.151677 Jingyun Zhang , Yumeng Liu , Zhen Jia , ShitaoYu , Shiwei Liu , Lu Li , Qiong Wu , Hailong Yu , Yuxiang Liu , Xiaoqing Jiang , Yue Liu , Chao Xu
|
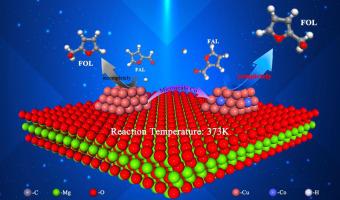
Cu-based catalysts have been widely studied as selectively hydrogenated furfural. However, due to the weak hydrogenation activity of the mono-metal copper-based catalyst, the interaction with furfural is usually weak, and the accessibility of the active site is severely limited. A micro-doping strategy was proposed in this study to solve these problems. Trace amounts of cobalt metal are doped into copper using equal volume impregnation. Compared with the Cu/MgO catalyst, the bimetallic CuCo/MgO-0.04 catalyst changes metal particles' size and valence and regulates the catalyst surface's electronic environment. Electron transfer occurs between Co and Cu, and many active sites are generated for adsorption and activation of C
中文翻译:

铜钴双金属催化剂将糠醛选择性加氢制糠醇
Cu 基催化剂作为选择性氢化糠醛已被广泛研究。然而,由于单金属铜基催化剂的氢化活性较弱,与糠醛的相互作用通常较弱,活性位点的可及性受到严重限制。本研究提出了一种微掺杂策略来解决这些问题。使用等体积浸渍将痕量的钴金属掺杂到铜中。与 Cu/MgO 催化剂相比,双金属 CuCo/MgO-0.04 催化剂改变了金属颗粒的尺寸和价,并调节了催化剂表面的电子环境。Co 和 Cu 之间发生电子转移,产生许多活性位点用于吸附和活化 CO 双键,并与相邻的 H 质子发生氢化反应,以提高糠醇的选择性。CuCo/MgO-0.04 催化剂具有优异的催化性能,糠醛转化率为 100%,糠醛醇选择性为 99.4%。结合实验数据、动力学研究和理论计算,证实了掺杂痕量金属钴的催化剂中 d 带中心上移,优化了糠醛的吸附能和构型,更有效地降低了反应势垒。本研究提供了一种策略,以支持高度分散的 Cu 纳米颗粒与少量 Co 金属的部分掺杂。它在选择性加氢反应中实现了优异的催化性能和化学选择性。
更新日期:2024-05-03
中文翻译:

铜钴双金属催化剂将糠醛选择性加氢制糠醇
Cu 基催化剂作为选择性氢化糠醛已被广泛研究。然而,由于单金属铜基催化剂的氢化活性较弱,与糠醛的相互作用通常较弱,活性位点的可及性受到严重限制。本研究提出了一种微掺杂策略来解决这些问题。使用等体积浸渍将痕量的钴金属掺杂到铜中。与 Cu/MgO 催化剂相比,双金属 CuCo/MgO-0.04 催化剂改变了金属颗粒的尺寸和价,并调节了催化剂表面的电子环境。Co 和 Cu 之间发生电子转移,产生许多活性位点用于吸附和活化 CO 双键,并与相邻的 H 质子发生氢化反应,以提高糠醇的选择性。CuCo/MgO-0.04 催化剂具有优异的催化性能,糠醛转化率为 100%,糠醛醇选择性为 99.4%。结合实验数据、动力学研究和理论计算,证实了掺杂痕量金属钴的催化剂中 d 带中心上移,优化了糠醛的吸附能和构型,更有效地降低了反应势垒。本研究提供了一种策略,以支持高度分散的 Cu 纳米颗粒与少量 Co 金属的部分掺杂。它在选择性加氢反应中实现了优异的催化性能和化学选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号