当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Tetra-Substituted 3-Hydroxyphthalide Esters by Isothiourea-Catalysed Acylative Dynamic Kinetic Resolution
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-05-07 , DOI: 10.1002/anie.202402909
Shubham K Agrawal 1 , Pankaj K Majhi 1 , Alister S Goodfellow 1 , Raj K Tak 1 , David B Cordes 1 , Aidan P McKay 1 , Kevin Kasten 1 , Michael Bühl 1 , Andrew D Smith 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-05-07 , DOI: 10.1002/anie.202402909
Shubham K Agrawal 1 , Pankaj K Majhi 1 , Alister S Goodfellow 1 , Raj K Tak 1 , David B Cordes 1 , Aidan P McKay 1 , Kevin Kasten 1 , Michael Bühl 1 , Andrew D Smith 1
Affiliation
|
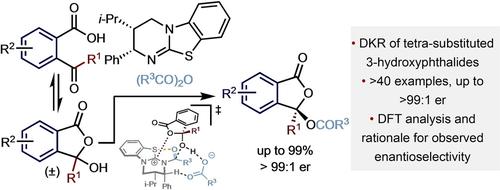
The enantioselective isothiourea-catalysed acylative dynamic kinetic resolution of tetra-substituted 3-hydroxyphthalide derivatives using (2S,3R)-HyperBTM (5 mol %) as the catalyst (>40 examples in total) is reported. A diverse range of anhydrides, including those derived from bioactive and pharmaceutically relevant acids, can be tolerated in this process with the origin of enantioselectivity probed using DFT analysis.
中文翻译:

异硫脲催化酰化动态动力学拆分合成四取代 3-羟基苯酞酯
报道了使用( 2S , 3R )-HyperBTM(5mol%)作为催化剂(总共%3E40个实例)对映选择性异硫脲催化的四取代3-羟基苯酞衍生物的酰化动态动力学拆分。在此过程中可以耐受多种酸酐,包括衍生自生物活性和药学相关酸的酸酐,并使用 DFT 分析探测对映选择性的起源。
更新日期:2024-05-07
中文翻译:

异硫脲催化酰化动态动力学拆分合成四取代 3-羟基苯酞酯
报道了使用( 2S , 3R )-HyperBTM(5mol%)作为催化剂(总共%3E40个实例)对映选择性异硫脲催化的四取代3-羟基苯酞衍生物的酰化动态动力学拆分。在此过程中可以耐受多种酸酐,包括衍生自生物活性和药学相关酸的酸酐,并使用 DFT 分析探测对映选择性的起源。

































 京公网安备 11010802027423号
京公网安备 11010802027423号