当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient, Scalable Synthesis of Functionalized Pyrrolo[2,1-f][1,2,4]triazines
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-07 , DOI: 10.1021/acs.oprd.4c00116 Shawn K. Pack 1 , Lei Feng 2 , Ce Chen 2 , Zhongzhen Li 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-07 , DOI: 10.1021/acs.oprd.4c00116 Shawn K. Pack 1 , Lei Feng 2 , Ce Chen 2 , Zhongzhen Li 2
Affiliation
|
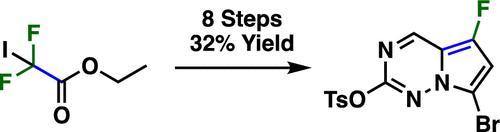
An efficient, scalable synthesis of functionalized 5-fluoropyrrolo[2,1-f][1,2,4]triazines was developed from readily available starting materials. The synthesis is highlighted by a safe, Cu mediated difluoroacetate addition to vinyl TMS, selective formylation of a 3-fluoropyrrole, and sequential imine formation followed by cyclization to the triazine core. Addition of other electrophiles to 3-fluoropyrrole was also shown to be equally selective.
中文翻译:

高效、可规模化合成功能化吡咯并[2,1-f][1,2,4]三嗪
利用现成的起始材料开发了一种高效、可规模化的官能化 5-氟吡咯并[2,1-f][1,2,4]三嗪合成方法。该合成的特点是安全、铜介导的二氟乙酸加成到乙烯基 TMS、3-氟吡咯的选择性甲酰化以及顺序亚胺形成,然后环化为三嗪核心。向 3-氟吡咯添加其他亲电子试剂也显示出同样的选择性。
更新日期:2024-05-07
中文翻译:

高效、可规模化合成功能化吡咯并[2,1-f][1,2,4]三嗪
利用现成的起始材料开发了一种高效、可规模化的官能化 5-氟吡咯并[2,1-f][1,2,4]三嗪合成方法。该合成的特点是安全、铜介导的二氟乙酸加成到乙烯基 TMS、3-氟吡咯的选择性甲酰化以及顺序亚胺形成,然后环化为三嗪核心。向 3-氟吡咯添加其他亲电子试剂也显示出同样的选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号