当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adaptive structural modification of Zr-based MOF-808 via solvent and ligand engineering for enhanced fluoride ion adsorption efficiency
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-05-03 , DOI: 10.1016/j.seppur.2024.127731 Yubo Pan , Linrui Li , Baogang Yang , Guangwei Ji , Zhiren Zhao , Hongling Zhang , Fengyun Wang , Mingzhu Xia , Yu Tao
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-05-03 , DOI: 10.1016/j.seppur.2024.127731 Yubo Pan , Linrui Li , Baogang Yang , Guangwei Ji , Zhiren Zhao , Hongling Zhang , Fengyun Wang , Mingzhu Xia , Yu Tao
|
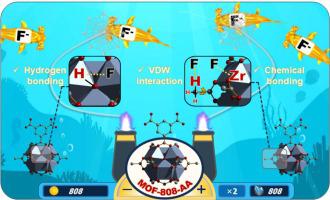
The rapid development of industries such as new energy and photovoltaic has led to the generation of a large amount of industrial fluorine wastewater, posing serious threats to water environment and human health. To address this issue, MOF-808 adsorbent was synthesized by controlling the synthesis conditions and modifying the ligand structure. Batch adsorption experiments were conducted by varying experimental conditions, including type of adsorbent, pH, wastewater concentration, adsorption time, temperature, and interfering anions. The adsorption behavior of MOF-808-AA towards fluoride ions was well fitted by the Elovich non-linear kinetic model, as well as the Freundlich and Temkin non-linear isotherm models, indicating that the adsorption behavior was influenced by multiple mechanisms other than just intraparticle diffusion. Thermodynamic results suggested a spontaneous exothermic monolayer chemical adsorption process. Under the conditions of pH 6.0 and T = 298 K, MOF-808-AA exhibited a maximum adsorption capacity of 84.65 mg/g for fluoride ions. Characterization techniques, as well as quantum chemical analysis was employed to analyze the adsorption behavior and predict the reaction sites. The potential mechanism can be summarized as the formation of new bonds between MOF-808-AA and the adsorbed fluoride ions, as well as the weak intermolecular interactions such as hydrogen bonding and vdW forces. Furthermore, MOF-808-AA demonstrated satisfactory reusability and excellent chemical stability during the cyclic process. These findings suggest the potential application of MOF-808-AA for removing fluoride ions from wastewater.
中文翻译:

通过溶剂和配体工程对锆基 MOF-808 进行自适应结构修饰以提高氟离子吸附效率
新能源、光伏等产业的快速发展导致大量工业含氟废水的产生,对水环境和人类健康构成严重威胁。为了解决这个问题,通过控制合成条件和修饰配体结构合成了MOF-808吸附剂。通过不同的实验条件进行批量吸附实验,包括吸附剂类型、pH、废水浓度、吸附时间、温度和干扰阴离子。 MOF-808-AA 对氟离子的吸附行为与 Elovich 非线性动力学模型以及 Freundlich 和 Temkin 非线性等温线模型很好地拟合,表明吸附行为除了受多种机制的影响外,还受到多种机制的影响。颗粒内扩散。热力学结果表明自发放热的单分子层化学吸附过程。在pH 6.0和T=298 K的条件下,MOF-808-AA对氟离子的最大吸附容量为84.65 mg/g。采用表征技术以及量子化学分析来分析吸附行为并预测反应位点。潜在的机制可以概括为MOF-808-AA与吸附的氟离子之间形成新的键,以及弱的分子间相互作用,例如氢键和vdW力。此外,MOF-808-AA在循环过程中表现出令人满意的可重复使用性和优异的化学稳定性。这些发现表明 MOF-808-AA 在去除废水中的氟离子方面具有潜在的应用前景。
更新日期:2024-05-03
中文翻译:

通过溶剂和配体工程对锆基 MOF-808 进行自适应结构修饰以提高氟离子吸附效率
新能源、光伏等产业的快速发展导致大量工业含氟废水的产生,对水环境和人类健康构成严重威胁。为了解决这个问题,通过控制合成条件和修饰配体结构合成了MOF-808吸附剂。通过不同的实验条件进行批量吸附实验,包括吸附剂类型、pH、废水浓度、吸附时间、温度和干扰阴离子。 MOF-808-AA 对氟离子的吸附行为与 Elovich 非线性动力学模型以及 Freundlich 和 Temkin 非线性等温线模型很好地拟合,表明吸附行为除了受多种机制的影响外,还受到多种机制的影响。颗粒内扩散。热力学结果表明自发放热的单分子层化学吸附过程。在pH 6.0和T=298 K的条件下,MOF-808-AA对氟离子的最大吸附容量为84.65 mg/g。采用表征技术以及量子化学分析来分析吸附行为并预测反应位点。潜在的机制可以概括为MOF-808-AA与吸附的氟离子之间形成新的键,以及弱的分子间相互作用,例如氢键和vdW力。此外,MOF-808-AA在循环过程中表现出令人满意的可重复使用性和优异的化学稳定性。这些发现表明 MOF-808-AA 在去除废水中的氟离子方面具有潜在的应用前景。































 京公网安备 11010802027423号
京公网安备 11010802027423号