当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In(OTf)3 promoted sonochemical approach to 3-(2-chloropyrimidin-4-yl)indoles: Their in silico and in vitro evaluation against SIRT1
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2024-04-26 , DOI: 10.1016/j.molstruc.2024.138471 Hemalatha Kotakommula , Vaishnavi Chintala , Satya Sree Nannapaneni , Naresh Kumar Katari , Ravikumar Kapavarapu , Manojit Pal
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2024-04-26 , DOI: 10.1016/j.molstruc.2024.138471 Hemalatha Kotakommula , Vaishnavi Chintala , Satya Sree Nannapaneni , Naresh Kumar Katari , Ravikumar Kapavarapu , Manojit Pal
|
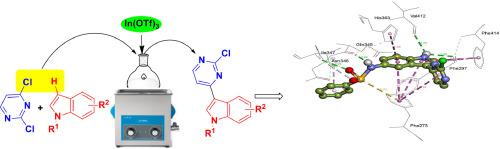
We report exploring the 3-(2-chloropyrimidin-4-yl)indole framework for the identification of new inhibitors of SIRT1. A library of compounds based on this framework was synthesized an ultrasound assisted heteroarylation approach. The methodology involved In(OTf) mediated CC bond forming reaction between 2,4-dichloropyrimidine and indoles to give the desired products in good to acceptable yields. The regioselectivity of this approach appeared to be a key feature as the methodology involved sonochemical replacement of the (i) C-3 hydrogen of indole and (ii) C-4 chloro group of the pyrimidine ring at the same time. Its synthetic application was demonstrated by converting one of the synthesized compounds into the naturally occurring alkaloid . The docking of 3-(2-chloropyrimidin-4-yl)indoles prepared showed promising interactions with SIRT1 in addition to probable selectivity towards this protein over SIRT2. A majority of compounds participated in H-bond interaction with GLN345 residue mainly through their indole “NH” moiety. However, higher binding affinities were observed for compounds possessing a sulfonamide moiety at C-5 position of the indole ring. Correlating the findings of studies a number of synthesized compounds showed inhibition of SIRT1 in vitro. With the inhibition > 70 % the compound and were identified as initial hit molecules when emerged as most promising among them. The SAR (Structure-Activity-Relationship) analysis also suggested that a sulfonamide moiety at C-5 position was beneficial for the high SIRT1 inhibition whereas a free NH moiety of the indole ring was essential for activity. Based on and in vitro studies including ADME predictions the compound appeared as a key agent for further pharmacological evaluation.
中文翻译:

In(OTf)3 促进了 3-(2-氯嘧啶-4-基)吲哚的声化学方法:针对 SIRT1 的计算机和体外评估
我们报告探索 3-(2-氯嘧啶-4-基)吲哚框架来鉴定新的 SIRT1 抑制剂。通过超声辅助杂芳基化方法合成了基于该框架的化合物库。该方法涉及 In(OTf) 介导的 2,4-二氯嘧啶和吲哚之间的 CC 键形成反应,以良好到可接受的收率得到所需产物。该方法的区域选择性似乎是一个关键特征,因为该方法涉及同时声化学取代 (i) 吲哚的 C-3 氢和 (ii) 嘧啶环的 C-4 氯基团。通过将一种合成化合物转化为天然存在的生物碱,证明了其合成应用。所制备的 3-(2-氯嘧啶-4-基)吲哚对接显示出与 SIRT1 的良好相互作用,此外,该蛋白相对于 SIRT2 可能具有选择性。大多数化合物主要通过其吲哚“NH”部分参与与 GLN345 残基的氢键相互作用。然而,对于在吲哚环的 C-5 位具有磺酰胺部分的化合物,观察到更高的结合亲和力。与研究结果相关联,许多合成化合物在体外显示出对 SIRT1 的抑制作用。该化合物的抑制率> 70%,当成为其中最有前途的分子时,被确定为最初的热门分子。 SAR(结构-活性-关系)分析还表明,C-5 位的磺酰胺部分有利于 SIRT1 的高抑制,而吲哚环的游离 NH 部分对于活性至关重要。根据包括 ADME 预测在内的体外研究,该化合物似乎是进一步药理学评估的关键药物。
更新日期:2024-04-26
中文翻译:

In(OTf)3 促进了 3-(2-氯嘧啶-4-基)吲哚的声化学方法:针对 SIRT1 的计算机和体外评估
我们报告探索 3-(2-氯嘧啶-4-基)吲哚框架来鉴定新的 SIRT1 抑制剂。通过超声辅助杂芳基化方法合成了基于该框架的化合物库。该方法涉及 In(OTf) 介导的 2,4-二氯嘧啶和吲哚之间的 CC 键形成反应,以良好到可接受的收率得到所需产物。该方法的区域选择性似乎是一个关键特征,因为该方法涉及同时声化学取代 (i) 吲哚的 C-3 氢和 (ii) 嘧啶环的 C-4 氯基团。通过将一种合成化合物转化为天然存在的生物碱,证明了其合成应用。所制备的 3-(2-氯嘧啶-4-基)吲哚对接显示出与 SIRT1 的良好相互作用,此外,该蛋白相对于 SIRT2 可能具有选择性。大多数化合物主要通过其吲哚“NH”部分参与与 GLN345 残基的氢键相互作用。然而,对于在吲哚环的 C-5 位具有磺酰胺部分的化合物,观察到更高的结合亲和力。与研究结果相关联,许多合成化合物在体外显示出对 SIRT1 的抑制作用。该化合物的抑制率> 70%,当成为其中最有前途的分子时,被确定为最初的热门分子。 SAR(结构-活性-关系)分析还表明,C-5 位的磺酰胺部分有利于 SIRT1 的高抑制,而吲哚环的游离 NH 部分对于活性至关重要。根据包括 ADME 预测在内的体外研究,该化合物似乎是进一步药理学评估的关键药物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号