Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immobilized Dipeptidase in Manganese Ion-Loaded Polyethylenimine-Induced Calcium Phosphate Nanocrystals for Carnosine Synthesis
Langmuir ( IF 3.7 ) Pub Date : 2024-05-02 , DOI: 10.1021/acs.langmuir.4c00759 Yujia Liu 1 , Jie Yu 1 , Yan Sun 1
Langmuir ( IF 3.7 ) Pub Date : 2024-05-02 , DOI: 10.1021/acs.langmuir.4c00759 Yujia Liu 1 , Jie Yu 1 , Yan Sun 1
Affiliation
|
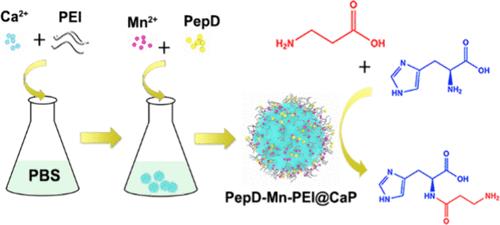
Carnosine is a natural bioactive dipeptide with important physiological functions widely used in food and medicine. Dipeptidase (PepD) from Serratia marcescens can catalyze the reverse hydrolytic reaction of β-alanine with l-histidine to synthesize carnosine in the presence of Mn2+. However, it remains challenging to practice carnosine biosynthesis due to the low activity and high cost of the enzyme. Therefore, the development of biocatalysts with high activity and stability is of significance for carnosine synthesis. Here, we proposed to chelate Mn2+ to polyethylenimine (PEI) that induced rapid formation of calcium phosphate nanocrystals (CaP), and Mn-PEI@CaP was used for PepD immobilization via electrostatic interaction. Mn-PEI@CaP as the carrier enhanced the stability of the immobilized enzyme. Moreover, Mn2+ loaded in the carrier acted as an in situ activator of the immobilized PepD for facilitating the biocatalytic process of carnosine synthesis. The as-prepared immobilized enzyme (PepD-Mn-PEI@CaP) kept similar activity with free PepD plus Mn2+ (activity recovery, 102.5%), while exhibiting elevated thermal stability and pH tolerance. Moreover, it exhibited about two times faster carnosine synthesis than the free PepD system. PepD-Mn-PEI@CaP retained 86.8% of the original activity after eight cycles of batch catalysis without the addition of free Mn2+ ions during multiple cycles. This work provides a new strategy for the co-immobilization of PepD and Mn2+, which greatly improves the operability of the biocatalysis and demonstrates the potential of the immobilized PepD system for efficient carnosine synthesis.
中文翻译:

锰离子负载聚乙烯亚胺诱导的磷酸钙纳米晶体中固定化二肽酶用于肌肽合成
肌肽是一种天然生物活性二肽,具有重要的生理功能,广泛应用于食品和医药领域。粘质沙雷氏菌的二肽酶(PepD)在Mn 2+存在下可催化β-丙氨酸与l-组氨酸的逆水解反应合成肌肽。然而,由于酶的活性低且成本高,实践肌肽生物合成仍然具有挑战性。因此,开发具有高活性和稳定性的生物催化剂对于肌肽合成具有重要意义。在这里,我们提出将 Mn 2+螯合到聚乙烯亚胺(PEI)上,诱导磷酸钙纳米晶体(CaP)的快速形成,并且 Mn-PEI@CaP 通过静电相互作用用于 PepD 固定化。 Mn-PEI@CaP作为载体增强了固定化酶的稳定性。此外,负载在载体中的Mn 2+作为固定化PepD的原位活化剂,促进肌肽合成的生物催化过程。所制备的固定化酶 (PepD-Mn-PEI@CaP) 与游离 PepD 加 Mn 2+保持相似的活性(活性回收率,102.5%),同时表现出较高的热稳定性和 pH 耐受性。此外,它的肌肽合成速度比游离 PepD 系统快两倍。 PepD-Mn-PEI@CaP 在八个循环的批量催化后保留了原始活性的 86.8%,并且在多个循环中不添加游离 Mn 2+离子。这项工作为PepD和Mn 2+的共固定化提供了新的策略,极大地提高了生物催化的可操作性,并展示了固定化PepD系统在高效肌肽合成中的潜力。
更新日期:2024-05-02
中文翻译:

锰离子负载聚乙烯亚胺诱导的磷酸钙纳米晶体中固定化二肽酶用于肌肽合成
肌肽是一种天然生物活性二肽,具有重要的生理功能,广泛应用于食品和医药领域。粘质沙雷氏菌的二肽酶(PepD)在Mn 2+存在下可催化β-丙氨酸与l-组氨酸的逆水解反应合成肌肽。然而,由于酶的活性低且成本高,实践肌肽生物合成仍然具有挑战性。因此,开发具有高活性和稳定性的生物催化剂对于肌肽合成具有重要意义。在这里,我们提出将 Mn 2+螯合到聚乙烯亚胺(PEI)上,诱导磷酸钙纳米晶体(CaP)的快速形成,并且 Mn-PEI@CaP 通过静电相互作用用于 PepD 固定化。 Mn-PEI@CaP作为载体增强了固定化酶的稳定性。此外,负载在载体中的Mn 2+作为固定化PepD的原位活化剂,促进肌肽合成的生物催化过程。所制备的固定化酶 (PepD-Mn-PEI@CaP) 与游离 PepD 加 Mn 2+保持相似的活性(活性回收率,102.5%),同时表现出较高的热稳定性和 pH 耐受性。此外,它的肌肽合成速度比游离 PepD 系统快两倍。 PepD-Mn-PEI@CaP 在八个循环的批量催化后保留了原始活性的 86.8%,并且在多个循环中不添加游离 Mn 2+离子。这项工作为PepD和Mn 2+的共固定化提供了新的策略,极大地提高了生物催化的可操作性,并展示了固定化PepD系统在高效肌肽合成中的潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号