当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial hydrogen bond effect between CeO2 and g-C3N4 boosts conversion to adipic acid from aerobic oxidation of cyclohexane
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-05-04 , DOI: 10.1016/j.cej.2024.151829 Kexin Li , Bin He , Ruirui Wang , Ruiyi Yan , Ruirui Zhang , Ruixia Liu
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-05-04 , DOI: 10.1016/j.cej.2024.151829 Kexin Li , Bin He , Ruirui Wang , Ruiyi Yan , Ruirui Zhang , Ruixia Liu
|
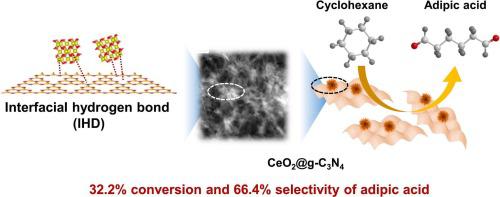
The selective oxidation reaction of cyclohexane is one of the typical activated sp3 C-H reactions, and its product adipic acid (AA) is the indispensable raw material used to produce nylon. However, subjecting to the inertness of C-H bond and the great reactivity of AA, the targeted transformation of cyclohexane remains a challenge. Here, the interfacial hydrogen bond (IHD) was developed by anchoring CeO2 on graphite carbon nitride (g-C3 N4 ) surface, which could boost targeted acquisition adipic acid from aerobic oxidation of cyclohexane. The results indicated that the IHD effect can be flexibly regulated by changing the ratio of Ce3+ /Ce4+ , further optimizing the properties of surface OH species and Lewis acid sites. Under the synergistic effect of Ce3+ /Ce4+ and Lewis acid sites on reaction interface, the glorious performance (32.2% conversion of cyclohexane with 66.4% selectivity) was obtained over the as-prepared CeO2 /g-C3 N4 IHD system. In addition, the interfacial adsorption properties of cyclohexane were explored by the virtue of in situ infrared spectroscopy, revealing the IHD effect could tune the adsorption behavior of cyclohexanone and further improve the selectivity of AA. This work disclosed that engineering the interface hydrogen bond effect between CeO2 and g-C3 N4 provided feasible strategies to obtain excellent catalyst for cyclohexane oxidation.
中文翻译:

CeO2 和 g-C3N4 之间的界面氢键效应促进了环己烷的有氧氧化转化为己二酸
环己烷的选择性氧化反应是典型的活化 sp3 C-H 反应之一,其产物己二酸 (AA) 是生产尼龙不可缺少的原料。然而,受制于 C-H 键的惰性和 AA 的巨大反应性,环己烷的靶向转化仍然是一个挑战。在这里,界面氢键 (IHD) 是通过将 CeO2 锚定在石墨氮化碳 (g-C3N4) 表面上来开发的,这可以促进环己烷有氧氧化对己二酸的靶向捕获。结果表明,通过改变 Ce3+/Ce4+ 的比例,可以灵活地调节 IHD 效应,进一步优化表面 OH 种类和路易斯酸位点的性质。在 Ce3+/Ce4+ 和 Lewis 酸位点对反应界面的协同作用下,与制备的 CeO2/g-C3N4 IHD 系统相比,获得了优异的性能 (环己烷转化率 32.2%,选择性为 66.4%)。此外,利用原位红外光谱探究了环己烷的界面吸附特性,揭示了 IHD 效应可以调节环己酮的吸附行为,进一步提高 AA 的选择性。这项工作揭示了 CeO2 和 g-C3N4 之间的界面氢键效应的工程设计为获得优良的环己烷氧化催化剂提供了可行的策略。
更新日期:2024-05-04
中文翻译:

CeO2 和 g-C3N4 之间的界面氢键效应促进了环己烷的有氧氧化转化为己二酸
环己烷的选择性氧化反应是典型的活化 sp3 C-H 反应之一,其产物己二酸 (AA) 是生产尼龙不可缺少的原料。然而,受制于 C-H 键的惰性和 AA 的巨大反应性,环己烷的靶向转化仍然是一个挑战。在这里,界面氢键 (IHD) 是通过将 CeO2 锚定在石墨氮化碳 (g-C3N4) 表面上来开发的,这可以促进环己烷有氧氧化对己二酸的靶向捕获。结果表明,通过改变 Ce3+/Ce4+ 的比例,可以灵活地调节 IHD 效应,进一步优化表面 OH 种类和路易斯酸位点的性质。在 Ce3+/Ce4+ 和 Lewis 酸位点对反应界面的协同作用下,与制备的 CeO2/g-C3N4 IHD 系统相比,获得了优异的性能 (环己烷转化率 32.2%,选择性为 66.4%)。此外,利用原位红外光谱探究了环己烷的界面吸附特性,揭示了 IHD 效应可以调节环己酮的吸附行为,进一步提高 AA 的选择性。这项工作揭示了 CeO2 和 g-C3N4 之间的界面氢键效应的工程设计为获得优良的环己烷氧化催化剂提供了可行的策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号