当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Fe(II) bio-oxidation rate and alkali control on schwertmannite microstructure and adsorption of oxyanions: Characteristics, performance and mechanism
Science of the Total Environment ( IF 8.2 ) Pub Date : 2024-04-27 , DOI: 10.1016/j.scitotenv.2024.172844 Feng Jiang 1 , Chao Xue 1 , Lijuan Zeng 1 , Yanjie Zheng 1 , Yaozhong Wang 1 , Xiaohu Jin 1 , Xiaoyun Yi 2 , Zhi Dang 2
Science of the Total Environment ( IF 8.2 ) Pub Date : 2024-04-27 , DOI: 10.1016/j.scitotenv.2024.172844 Feng Jiang 1 , Chao Xue 1 , Lijuan Zeng 1 , Yanjie Zheng 1 , Yaozhong Wang 1 , Xiaohu Jin 1 , Xiaoyun Yi 2 , Zhi Dang 2
Affiliation
|
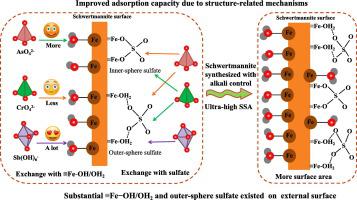
Schwertmannite has attracted increasing interest for its excellent sorption of oxyanions such as AsO4 3− , CrO4 2− , and Sb(OH)6 − . Controlling biomineralization by adjusting the Fe(II) oxidation rate and implementing alkali control can enhance the yield and adsorption performance of schwertmannite. However, the adsorption improvement mechanism is still unclear. The morphology, crystallinity, specific surface area (SSA) and oxyanion adsorption of schwertmannite synthesized with alkali control of solution pH and different Fe(II) oxidation rates were analyzed in this study. The differences in the adsorption mechanisms of As(V), Cr(VI) and Sb(V) on schwertmannite obtained under different synthesis conditions were also studied. Reducing the Fe(II) oxidation rate or maintaining the solution pH through alkali control significantly increased the SSA of schwertmannite and the proportion of outer-sphere sulfate. Alkali-controlled schwertmannite (Sch-C) exhibited superior As(V) and Sb(V) adsorption performance and slightly greater Cr(VI) adsorption than non-alkali-controlled schwertmannite. The As(V) and Sb(V) adsorption capacities of Sch-C greatly improved because the ultra-high SSA increased the surface hydroxyl content and reduced the passivation effect of amorphous precipitates on the mineral surface, allowing continuous sulfate exchange at inner mineral sites. An increased surface hydroxyl content had little effect on Cr(VI) adsorption, but an increased proportion of outer-sphere sulfate caused a slight increase in Cr(VI) adsorption. Sb(V) has a stronger hydroxyl exchange ability than As(V), but due to its octahedral structure, it exchanges only with outer-sphere sulfate on schwertmannite and hardly exchanges with inner-sphere sulfate.
中文翻译:

Fe(II)生物氧化速率和碱控制对施威特曼石微观结构和氧阴离子吸附的影响:特性、性能和机制
施韦特曼石因其对 AsO43−、CrO42− 和 Sb(OH)6− 等氧阴离子的出色吸附而引起了越来越多的关注。通过调节Fe(II)氧化速率和实施碱控制来控制生物矿化可以提高施威特曼石的产率和吸附性能。然而,吸附改善机制仍不清楚。本研究分析了碱控制溶液 pH 值和不同 Fe(II) 氧化速率合成的施威特曼石的形貌、结晶度、比表面积 (SSA) 和氧阴离子吸附。研究了不同合成条件下所得施威特曼石对As(V)、Cr(VI)和Sb(V)吸附机理的差异。降低 Fe(II) 氧化速率或通过碱控制维持溶液 pH 值可显着增加施威特曼石的 SSA 和外球硫酸盐的比例。碱控施威特曼石 (Sch-C) 表现出优于非碱控施威特曼石的 As(V) 和 Sb(V) 吸附性能,并且对 Cr(VI) 的吸附略强。 Sch-C的As(V)和Sb(V)吸附能力大大提高,因为超高的SSA增加了表面羟基含量,减少了矿物表面无定形沉淀物的钝化作用,允许内部矿物位点持续硫酸盐交换。表面羟基含量的增加对Cr(VI)吸附影响不大,但外层硫酸盐比例的增加导致Cr(VI)吸附略有增加。 Sb(V)比As(V)具有更强的羟基交换能力,但由于其八面体结构,仅与施威特曼石上的外层硫酸盐交换,几乎不与内层硫酸盐交换。
更新日期:2024-04-27
中文翻译:

Fe(II)生物氧化速率和碱控制对施威特曼石微观结构和氧阴离子吸附的影响:特性、性能和机制
施韦特曼石因其对 AsO43−、CrO42− 和 Sb(OH)6− 等氧阴离子的出色吸附而引起了越来越多的关注。通过调节Fe(II)氧化速率和实施碱控制来控制生物矿化可以提高施威特曼石的产率和吸附性能。然而,吸附改善机制仍不清楚。本研究分析了碱控制溶液 pH 值和不同 Fe(II) 氧化速率合成的施威特曼石的形貌、结晶度、比表面积 (SSA) 和氧阴离子吸附。研究了不同合成条件下所得施威特曼石对As(V)、Cr(VI)和Sb(V)吸附机理的差异。降低 Fe(II) 氧化速率或通过碱控制维持溶液 pH 值可显着增加施威特曼石的 SSA 和外球硫酸盐的比例。碱控施威特曼石 (Sch-C) 表现出优于非碱控施威特曼石的 As(V) 和 Sb(V) 吸附性能,并且对 Cr(VI) 的吸附略强。 Sch-C的As(V)和Sb(V)吸附能力大大提高,因为超高的SSA增加了表面羟基含量,减少了矿物表面无定形沉淀物的钝化作用,允许内部矿物位点持续硫酸盐交换。表面羟基含量的增加对Cr(VI)吸附影响不大,但外层硫酸盐比例的增加导致Cr(VI)吸附略有增加。 Sb(V)比As(V)具有更强的羟基交换能力,但由于其八面体结构,仅与施威特曼石上的外层硫酸盐交换,几乎不与内层硫酸盐交换。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号