当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the Mechanism of Tetrahydrofuran-Diol Formation through Os(VI)-Catalyzed Oxidative Cyclization of 5,6-Dihydroxyalkenes Ligated by Citric Acid
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-05-03 , DOI: 10.1021/acs.joc.4c00268
Aqeel A Hussein 1, 2 , Nadhir N A Jafar 2 , Yumiao Ma 3, 4
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-05-03 , DOI: 10.1021/acs.joc.4c00268
Aqeel A Hussein 1, 2 , Nadhir N A Jafar 2 , Yumiao Ma 3, 4
Affiliation
|
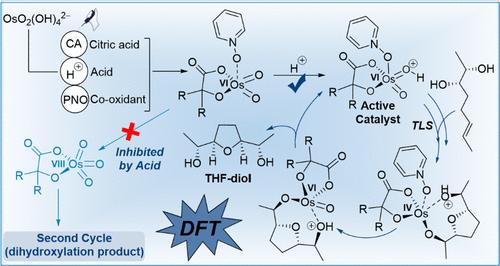
A computational study is reported here on the mechanism of tetrahydrofuran (THF)-diol formation from the Os(VI)-catalyzed oxidative cyclization of 5,6-dihydroxyalkene ligated with citric acid and in the presence of Bro̷nsted acid. Initiated by Os(VI) dioxo citrate formation, coordination of co-oxidant pyridine-N-oxide (PNO) and protonation of its oxo group generate the active catalyst. The catalytic cycle commences through successive steps, including dihydroxyalkene addition to the active catalyst in a concerted mechanism to form hexacoordinated alkoxy-protonated PNO-complexed Os(VI) bisglycolate as a turnover-limiting step (TLS), cyclization to Os(IV) THF-diolate, reoxidation to Os(VI) THF-diolate, and hydrolysis via a dissociative mechanism to furnish the THF-diol and regenerate the active species, sustaining the catalytic cycle through an Os(VI)/Os(IV) cycle. Despite the overall exergonic nature of catalytic cycle (ΔGrcycle = −45.0 kcal/mol), the TLS is accelerated by the formation of an open-valence 16-electron Os(VI) intermediate but decelerated by the undesired formation of a saturated/hexacoordinate 18-electron Os(VI) intermediate. Bro̷nsted acid plays crucial roles in the formation of Os(VI) citrate and the active catalyst, impediment of the second cycle, and the cyclization step. Additionally, besides its role as a co-oxidant, and in the presence of acid, PNO is found to assist the insertion of dihydroxyalkene and, importantly, in releasing the THF-diol to regenerate the active intermediate.
中文翻译:

阐明柠檬酸连接的 5,6-二羟基烯烃通过 Os(VI) 催化氧化环化形成四氢呋喃二醇的机制
本文报道了一项关于在布朗斯台德酸存在下,Os(VI) 催化的 5,6-二羟基烯烃与柠檬酸连接的氧化环化形成四氢呋喃 (THF)-二醇的机制的计算研究。由 Os(VI) 二氧代柠檬酸盐形成引发,共氧化剂吡啶-N-氧化物 (PNO) 的配位及其氧代基团的质子化产生活性催化剂。催化循环通过连续步骤开始,包括以协同机制将二羟基烯烃添加到活性催化剂中,形成六配位烷氧基质子化的 PNO 络合 Os(VI) 双乙醇酸酯作为周转限制步骤 (TLS),环化为 Os(IV) THF -二醇,再氧化为 Os(VI) THF-二醇,并通过解离机制水解以提供 THF-二醇并再生活性物质,通过 Os(VI)/Os(IV) 循环维持催化循环。尽管催化循环具有整体放能性质( ΔG r循环= -45.0 kcal/mol),但 TLS 通过开价 16 电子 Os(VI) 中间体的形成而加速,但由于不期望的饱和中间体的形成而减速。 /六配位18电子Os(VI)中间体。布朗斯台德酸在柠檬酸 Os(VI) 和活性催化剂的形成、第二循环的障碍和环化步骤中起着至关重要的作用。此外,除了作为助氧化剂的作用外,在酸存在的情况下,PNO 还被发现有助于二羟基烯烃的插入,更重要的是,有助于释放 THF-二醇以再生活性中间体。
更新日期:2024-05-03
中文翻译:

阐明柠檬酸连接的 5,6-二羟基烯烃通过 Os(VI) 催化氧化环化形成四氢呋喃二醇的机制
本文报道了一项关于在布朗斯台德酸存在下,Os(VI) 催化的 5,6-二羟基烯烃与柠檬酸连接的氧化环化形成四氢呋喃 (THF)-二醇的机制的计算研究。由 Os(VI) 二氧代柠檬酸盐形成引发,共氧化剂吡啶-N-氧化物 (PNO) 的配位及其氧代基团的质子化产生活性催化剂。催化循环通过连续步骤开始,包括以协同机制将二羟基烯烃添加到活性催化剂中,形成六配位烷氧基质子化的 PNO 络合 Os(VI) 双乙醇酸酯作为周转限制步骤 (TLS),环化为 Os(IV) THF -二醇,再氧化为 Os(VI) THF-二醇,并通过解离机制水解以提供 THF-二醇并再生活性物质,通过 Os(VI)/Os(IV) 循环维持催化循环。尽管催化循环具有整体放能性质( ΔG r循环= -45.0 kcal/mol),但 TLS 通过开价 16 电子 Os(VI) 中间体的形成而加速,但由于不期望的饱和中间体的形成而减速。 /六配位18电子Os(VI)中间体。布朗斯台德酸在柠檬酸 Os(VI) 和活性催化剂的形成、第二循环的障碍和环化步骤中起着至关重要的作用。此外,除了作为助氧化剂的作用外,在酸存在的情况下,PNO 还被发现有助于二羟基烯烃的插入,更重要的是,有助于释放 THF-二醇以再生活性中间体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号