Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-methyl Formamide Electrolyte Additive Enabling Highly Reversible Zn Anodes
Small ( IF 13.0 ) Pub Date : 2024-05-03 , DOI: 10.1002/smll.202400673 Peter Joseph Chimtali 1 , Xiya Yang 1 , Quan Zhou 1 , Shiqiang Wei 1 , Zeinab Mohamed 1 , Hassan Akhtar 1 , Aad Al-Mahgari 1 , Yuzhu Zhou 1 , Hanchen Xu 1 , Zijun Zhang 1 , Dengfeng Cao 1 , Shuangming Chen 1 , Kefu Zhu 1 , Xin Guo 1 , Hongwei Shou 1, 2 , Xiaojun Wu 1, 2 , Changda Wang 1 , Li Song 1, 3
Small ( IF 13.0 ) Pub Date : 2024-05-03 , DOI: 10.1002/smll.202400673 Peter Joseph Chimtali 1 , Xiya Yang 1 , Quan Zhou 1 , Shiqiang Wei 1 , Zeinab Mohamed 1 , Hassan Akhtar 1 , Aad Al-Mahgari 1 , Yuzhu Zhou 1 , Hanchen Xu 1 , Zijun Zhang 1 , Dengfeng Cao 1 , Shuangming Chen 1 , Kefu Zhu 1 , Xin Guo 1 , Hongwei Shou 1, 2 , Xiaojun Wu 1, 2 , Changda Wang 1 , Li Song 1, 3
Affiliation
|
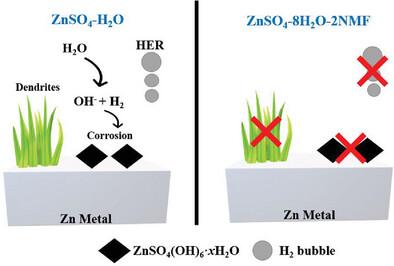
Parasitic side reactions and dendrites formation hinder the application of aqueous zinc ion batteries due to inferior cycling life and low reversibility. Against this background, N-methyl formamide (NMF), a multi-function electrolyte additive is applied to enhance the electrochemical performance. Studied via advanced synchrotron radiation spectroscopy and DFT calculations, the NMF additive simultaneously modifies the Zn2+ solvation structure and ensures uniform zinc deposition, thus suppressing both parasitic side reactions and dendrite formation. More importantly, an ultralong cycling life of 3115 h in the Zn||Zn symmetric cell at a current density of 0.5 mA cm−2 is achieved with the NMF additive. Practically, the Zn||PANI full cell utilizing NMF electrolyte shows better rate and cycling performance compared to the pristine ZnSO4 aqueous electrolyte. This work provides useful insights for the development of high-performance aqueous metal batteries.
中文翻译:

N-甲基甲酰胺电解质添加剂可实现高度可逆的 Zn 阳极
寄生副反应和枝晶形成由于循环寿命短和可逆性低,阻碍了水性锌离子电池的应用。在此背景下,应用多功能电解质添加剂 N-甲基甲酰胺 (NMF) 来增强电化学性能。通过先进的同步辐射光谱和 DFT 计算进行研究,NMF 添加剂同时改变了 Zn2+ 溶剂化结构并确保锌均匀沉积,从而抑制了寄生副反应和枝晶形成。更重要的是,Zn||电流密度为 0.5 mA cm-2 的 Zn 对称电池是通过 NMF 添加剂实现的。实际上,Zn||与原始的 ZnSO4 水性电解质相比,使用 NMF 电解质的 PANI 全电池显示出更好的倍率和循环性能。这项工作为高性能水系金属电池的开发提供了有益的见解。
更新日期:2024-05-03
中文翻译:

N-甲基甲酰胺电解质添加剂可实现高度可逆的 Zn 阳极
寄生副反应和枝晶形成由于循环寿命短和可逆性低,阻碍了水性锌离子电池的应用。在此背景下,应用多功能电解质添加剂 N-甲基甲酰胺 (NMF) 来增强电化学性能。通过先进的同步辐射光谱和 DFT 计算进行研究,NMF 添加剂同时改变了 Zn2+ 溶剂化结构并确保锌均匀沉积,从而抑制了寄生副反应和枝晶形成。更重要的是,Zn||电流密度为 0.5 mA cm-2 的 Zn 对称电池是通过 NMF 添加剂实现的。实际上,Zn||与原始的 ZnSO4 水性电解质相比,使用 NMF 电解质的 PANI 全电池显示出更好的倍率和循环性能。这项工作为高性能水系金属电池的开发提供了有益的见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号