当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site‐Selective Disulfide Modification of Proteins: Expanding Diversity beyond the Proteome
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-10-25 , DOI: 10.1002/chem.201602298 Seah Ling Kuan 1, 2 , Tao Wang 1, 3 , Tanja Weil 1, 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-10-25 , DOI: 10.1002/chem.201602298 Seah Ling Kuan 1, 2 , Tao Wang 1, 3 , Tanja Weil 1, 2
Affiliation
|
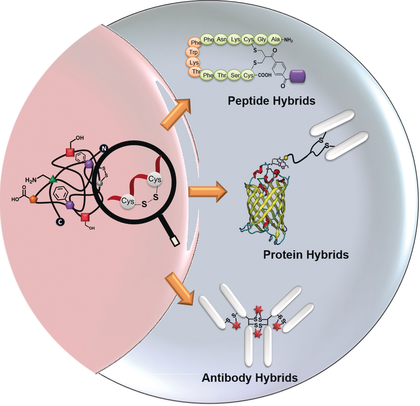
The synthetic transformation of polypeptides with molecular accuracy holds great promise for providing functional and structural diversity beyond the proteome. Consequently, the last decade has seen an exponential growth of site‐directed chemistry to install additional features into peptides and proteins even inside living cells. The disulfide rebridging strategy has emerged as a powerful tool for site‐selective modifications since most proteins contain disulfide bonds. In this Review, we present the chemical design, advantages and limitations of the disulfide rebridging reagents, while summarizing their relevance for synthetic customization of functional protein bioconjugates, as well as the resultant impact and advancement for biomedical applications.
中文翻译:

蛋白质的位点选择性二硫键修饰:将多样性扩展到蛋白质组之外
具有分子精度的多肽合成转化对于提供蛋白质组以外的功能和结构多样性具有巨大的前景。因此,在过去的十年里,定点化学技术呈指数级增长,将附加功能安装到肽和蛋白质中,甚至在活细胞内。由于大多数蛋白质都含有二硫键,二硫键重桥策略已成为位点选择性修饰的强大工具。在这篇综述中,我们介绍了二硫键重桥试剂的化学设计、优点和局限性,同时总结了它们与功能性蛋白质生物缀合物合成定制的相关性,以及对生物医学应用产生的影响和进步。
更新日期:2016-10-25
中文翻译:

蛋白质的位点选择性二硫键修饰:将多样性扩展到蛋白质组之外
具有分子精度的多肽合成转化对于提供蛋白质组以外的功能和结构多样性具有巨大的前景。因此,在过去的十年里,定点化学技术呈指数级增长,将附加功能安装到肽和蛋白质中,甚至在活细胞内。由于大多数蛋白质都含有二硫键,二硫键重桥策略已成为位点选择性修饰的强大工具。在这篇综述中,我们介绍了二硫键重桥试剂的化学设计、优点和局限性,同时总结了它们与功能性蛋白质生物缀合物合成定制的相关性,以及对生物医学应用产生的影响和进步。















































 京公网安备 11010802027423号
京公网安备 11010802027423号