当前位置:
X-MOL 学术
›
Biomass Bioenergy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics and thermodynamics in microwave-assisted transesterification of palm oil utilizing sulphonated bio-graphene catalysts for biodiesel production
Biomass & Bioenergy ( IF 5.8 ) Pub Date : 2024-05-01 , DOI: 10.1016/j.biombioe.2024.107236 Mohammed Abdillah Ahmad Farid , Siti Aminah Mohd Johari , Jacqueline Lease , Mohammad Ayoub , Yoshito Andou
Biomass & Bioenergy ( IF 5.8 ) Pub Date : 2024-05-01 , DOI: 10.1016/j.biombioe.2024.107236 Mohammed Abdillah Ahmad Farid , Siti Aminah Mohd Johari , Jacqueline Lease , Mohammad Ayoub , Yoshito Andou
|
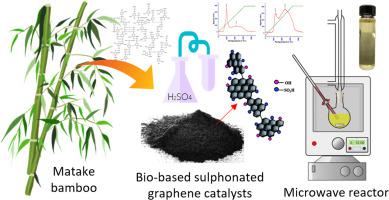
Enhanced reaction performance is what has piqued interest in using graphene-based catalysts in producing biodiesel. However, in-depth analyses were lacking from a kinetic and thermodynamic standpoint. This means that a well-designed experiment is necessary to determine the effect that the graphene catalysts have on the reaction rates and yields. This work addresses the kinetic and thermodynamic aspects of heterogeneously catalyzed transesterification using sulphonated biomass-derived graphene catalysts, i.e., bGO-SOH and brGO-SOH. It was accomplished using the pseudo-first order mechanism and the Eyring-Polanyi equation, with all data fitting satisfactorily in both models at the ideal reaction temperature of 353.15 K, resulting in R values of 0.9784 (bGO-SOH) and 0.9884 (brGO-SOH). The calculated activation energy () was determined to be 44.45 and 51.73 kJ mol1 for bGO- and brGO-SOH, respectively. Additionally, the Gibbs free energy (ΔG) values were determined at −38.09 and −31.81 kJ mol for bGO- and brGO-SOH, respectively. Meanwhile, the values for ΔH and ΔS were obtained as 41.69 kJ mol and 225.93 J mol K, respectively, and 48.96 kJ mol and 228.72 J mol K, respectively. These collective outcomes collectively signify the endothermic nature of the reaction and their spontaneity, particularly under elevated temperature conditions.
中文翻译:

利用磺化生物石墨烯催化剂微波辅助棕榈油酯交换反应生产生物柴油的动力学和热力学
增强的反应性能激起了人们对使用石墨烯基催化剂生产生物柴油的兴趣。然而,缺乏从动力学和热力学角度进行深入分析。这意味着需要精心设计的实验来确定石墨烯催化剂对反应速率和产率的影响。这项工作解决了使用磺化生物质衍生的石墨烯催化剂(即 bGO-SOH 和 brGO-SOH)进行非均相催化酯交换反应的动力学和热力学方面的问题。它是使用伪一阶机制和 Eyring-Polanyi 方程完成的,在 353.15 K 的理想反应温度下,所有数据在两个模型中均拟合良好,导致 R 值为 0.9784 (bGO-SOH) 和 0.9884 (brGO-苏)。 bGO-和 brGO-SOH 的计算活化能 () 分别为 44.45 和 51.73 kJ mol1。此外,bGO- 和 brGO-SOH 的吉布斯自由能 (ΔG) 值分别为 -38.09 和 -31.81 kJ mol。同时,ΔH 和 ΔS 的值分别为 41.69 kJ mol 和 225.93 J mol K,以及 48.96 kJ mol 和 228.72 J mol K。这些集体结果共同表明了反应的吸热性质及其自发性,特别是在高温条件下。
更新日期:2024-05-01
中文翻译:

利用磺化生物石墨烯催化剂微波辅助棕榈油酯交换反应生产生物柴油的动力学和热力学
增强的反应性能激起了人们对使用石墨烯基催化剂生产生物柴油的兴趣。然而,缺乏从动力学和热力学角度进行深入分析。这意味着需要精心设计的实验来确定石墨烯催化剂对反应速率和产率的影响。这项工作解决了使用磺化生物质衍生的石墨烯催化剂(即 bGO-SOH 和 brGO-SOH)进行非均相催化酯交换反应的动力学和热力学方面的问题。它是使用伪一阶机制和 Eyring-Polanyi 方程完成的,在 353.15 K 的理想反应温度下,所有数据在两个模型中均拟合良好,导致 R 值为 0.9784 (bGO-SOH) 和 0.9884 (brGO-苏)。 bGO-和 brGO-SOH 的计算活化能 () 分别为 44.45 和 51.73 kJ mol1。此外,bGO- 和 brGO-SOH 的吉布斯自由能 (ΔG) 值分别为 -38.09 和 -31.81 kJ mol。同时,ΔH 和 ΔS 的值分别为 41.69 kJ mol 和 225.93 J mol K,以及 48.96 kJ mol 和 228.72 J mol K。这些集体结果共同表明了反应的吸热性质及其自发性,特别是在高温条件下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号