当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Azepinoindoles and Oxepinoindoles through Brønsted-Acid-Catalyzed Cyclization of an In Situ Generated Dihydrospiroquinoline Intermediate
Organic Letters ( IF 4.9 ) Pub Date : 2024-05-01 , DOI: 10.1021/acs.orglett.4c01091 Rina Mahato 1 , Naveen Yadav 1 , Chinmoy Kumar Hazra 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-05-01 , DOI: 10.1021/acs.orglett.4c01091 Rina Mahato 1 , Naveen Yadav 1 , Chinmoy Kumar Hazra 1
Affiliation
|
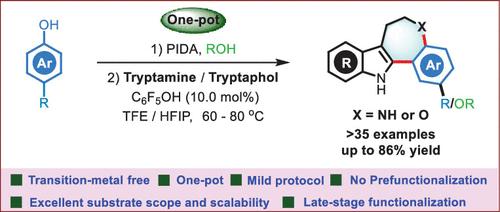
We have developed a straightforward and efficient synthetic protocol to produce 5,6,7,12-tetrahydrobenzo[2,3]azepino[4,5-b]indole and 7,12-dihydro-6H-benzo[2,3]oxepino[4,5-b]indole derivatives under mild conditions. This annulation process involves the intramolecular cyclization of the in situ generated ketimine moiety via the formation of dihydrospiroindolequinoline, which serves as a key intermediate in the reaction pathway. Several control experiments and spectroscopic studies were performed to elucidate the underlying reaction mechanism.
中文翻译:

通过布朗斯台德酸催化环化原位生成的二氢螺喹啉中间体合成氮杂吲哚和氧杂吲哚
我们开发了一种简单有效的合成方案来生产 5,6,7,12-四氢苯并[2,3]氮杂[4,5- b ]吲哚和 7,12-二氢-6 H-苯并[2,3] oxepino[4,5- b ]吲哚衍生物在温和条件下。该成环过程涉及通过形成二氢螺吲哚喹啉(作为反应途径中的关键中间体)原位生成的酮亚胺部分的分子内环化。进行了一些对照实验和光谱研究以阐明潜在的反应机制。
更新日期:2024-05-01
中文翻译:

通过布朗斯台德酸催化环化原位生成的二氢螺喹啉中间体合成氮杂吲哚和氧杂吲哚
我们开发了一种简单有效的合成方案来生产 5,6,7,12-四氢苯并[2,3]氮杂[4,5- b ]吲哚和 7,12-二氢-6 H-苯并[2,3] oxepino[4,5- b ]吲哚衍生物在温和条件下。该成环过程涉及通过形成二氢螺吲哚喹啉(作为反应途径中的关键中间体)原位生成的酮亚胺部分的分子内环化。进行了一些对照实验和光谱研究以阐明潜在的反应机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号