当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of α-Chiral Bicyclo[1.1.1]pentanes via Multicomponent Asymmetric Allylic Alkylation
Organic Letters ( IF 4.9 ) Pub Date : 2024-04-30 , DOI: 10.1021/acs.orglett.4c00902 Sergio Barbeira-Arán 1 , Irene Sánchez-Sordo 1 , Martín Fañanás-Mastral 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-04-30 , DOI: 10.1021/acs.orglett.4c00902 Sergio Barbeira-Arán 1 , Irene Sánchez-Sordo 1 , Martín Fañanás-Mastral 1
Affiliation
|
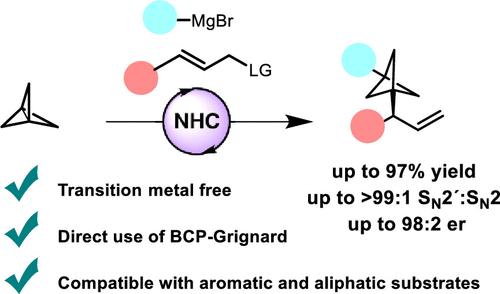
Bicyclo[1.1.1]pentanes (BCPs) have emerged as important structural motifs in drug design. However, asymmetric transformations that provide chiral BCPs bearing an adjacent stereocenter are still scarce. Here, we report a catalytic methodology for the enantioselective synthesis of α-chiral 1,3-difunctionalized BCPs from a three-component coupling of [1.1.1]propellane, a Grignard reagent, and an allylic phosphate. The reaction proceeds via the addition of the Grignard reagent to [1.1.1]propellane followed by an asymmetric N-heterocyclic carbene (NHC)-catalyzed allylic substitution of the resulting BCP–Grignard, providing a broad range of α-chiral BCPs with excellent levels of regioselectivity and enantioselectivity.
中文翻译:

通过多组分不对称烯丙基烷基化对映选择性合成α-手性双环[1.1.1]戊烷
双环[1.1.1]戊烷(BCP)已成为药物设计中的重要结构基序。然而,提供带有相邻立构中心的手性 BCP 的不对称变换仍然很少。在这里,我们报告了一种通过[1.1.1]丙烷、格氏试剂和烯丙磷酸酯的三组分偶联对映选择性合成α-手性1,3-双官能化BCP的催化方法。该反应通过将格氏试剂添加到[1.1.1]丙烷中进行,然后用不对称的N-杂环卡宾(NHC)催化生成的BCP-格氏试剂进行烯丙基取代,从而提供了多种具有优异性能的α-手性BCP。区域选择性和对映选择性水平。
更新日期:2024-04-30
中文翻译:

通过多组分不对称烯丙基烷基化对映选择性合成α-手性双环[1.1.1]戊烷
双环[1.1.1]戊烷(BCP)已成为药物设计中的重要结构基序。然而,提供带有相邻立构中心的手性 BCP 的不对称变换仍然很少。在这里,我们报告了一种通过[1.1.1]丙烷、格氏试剂和烯丙磷酸酯的三组分偶联对映选择性合成α-手性1,3-双官能化BCP的催化方法。该反应通过将格氏试剂添加到[1.1.1]丙烷中进行,然后用不对称的N-杂环卡宾(NHC)催化生成的BCP-格氏试剂进行烯丙基取代,从而提供了多种具有优异性能的α-手性BCP。区域选择性和对映选择性水平。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号