当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Remote Hydrosulfonamidation of Alkenes: Access to Primary N-Alkyl Sulfamides by the SuFEx Reaction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-05-02 , DOI: 10.1021/jacs.4c03283
Chuanqi Hou 1 , Zhenye Liu 2 , Lan Gan 1 , Wenzheng Fan 1 , Lin Huang 1 , Pinhong Chen 1 , Zheng Huang 1 , Guosheng Liu 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-05-02 , DOI: 10.1021/jacs.4c03283
Chuanqi Hou 1 , Zhenye Liu 2 , Lan Gan 1 , Wenzheng Fan 1 , Lin Huang 1 , Pinhong Chen 1 , Zheng Huang 1 , Guosheng Liu 1, 2
Affiliation
|
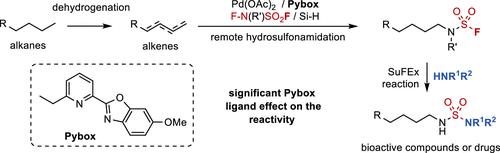
Herein, we establish a remote hydrosulfonamidation (HSA) of alkenes using palladium catalysis, where N-fluoro-N-(fluoro-sulfonyl)-carbamate with a sulfur(VI) fluoride moiety is demonstrated as a good amidation reagent. The anti-Markovnikov HSA reaction of terminal alkenes and the remote HSA of internal alkenes are achieved to efficiently yield primary N-alkyl-N-(fluorosulfonyl)-carbamates. In addition, this protocol enables the high-value utilization of alkane by combining the dehydrogenation process. The generated N-alkyl products exhibit a unique reactivity of sulfur(VI) fluorides, which can be directly transferred to N-alkyl sulfamides or amines via the sulfur(VI) fluoride exchange reaction, thereby streamlining their synthesis. Moreover, a (pyridyl) benzazole-type ligand proved to be vital for the excellent chemo- and regioselectivities.
中文翻译:

钯催化烯烃远程氢磺酰胺化:通过 SuFEx 反应获得伯 N-烷基磺酰胺
在此,我们利用钯催化建立了烯烃的远程氢磺酰胺化(HSA),其中具有硫(VI)氟化物部分的N-氟-N- (氟磺酰基)-氨基甲酸酯被证明是一种良好的酰胺化试剂。实现末端烯烃的反马尔可夫尼科夫HSA反应和内部烯烃的远程HSA反应,有效地生成伯N-烷基-N- (氟磺酰基)-氨基甲酸酯。此外,该方案通过结合脱氢过程实现烷烃的高值利用。生成的N-烷基产物表现出独特的氟化硫(VI)反应活性,可以通过氟化硫(VI)交换反应直接转化为N-烷基磺酰胺或胺,从而简化其合成。此外,(吡啶基)苯并唑型配体被证明对于优异的化学和区域选择性至关重要。
更新日期:2024-05-02
中文翻译:

钯催化烯烃远程氢磺酰胺化:通过 SuFEx 反应获得伯 N-烷基磺酰胺
在此,我们利用钯催化建立了烯烃的远程氢磺酰胺化(HSA),其中具有硫(VI)氟化物部分的N-氟-N- (氟磺酰基)-氨基甲酸酯被证明是一种良好的酰胺化试剂。实现末端烯烃的反马尔可夫尼科夫HSA反应和内部烯烃的远程HSA反应,有效地生成伯N-烷基-N- (氟磺酰基)-氨基甲酸酯。此外,该方案通过结合脱氢过程实现烷烃的高值利用。生成的N-烷基产物表现出独特的氟化硫(VI)反应活性,可以通过氟化硫(VI)交换反应直接转化为N-烷基磺酰胺或胺,从而简化其合成。此外,(吡啶基)苯并唑型配体被证明对于优异的化学和区域选择性至关重要。




































 京公网安备 11010802027423号
京公网安备 11010802027423号