当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coprecipitation of Fe/Cr Hydroxides at Organic–Water Interfaces: Functional Group Richness and (De)protonation Control Amounts and Compositions of Coprecipitates
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-05-02 , DOI: 10.1021/acs.est.4c01245 Yandi Hu 1, 2 , Xulin Jiang 1 , Suona Zhang 1 , Dawei Cai 1 , Zehao Zhou 1 , Chuan Liu 1 , Xiaobing Zuo 3 , Sang Soo Lee 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-05-02 , DOI: 10.1021/acs.est.4c01245 Yandi Hu 1, 2 , Xulin Jiang 1 , Suona Zhang 1 , Dawei Cai 1 , Zehao Zhou 1 , Chuan Liu 1 , Xiaobing Zuo 3 , Sang Soo Lee 4
Affiliation
|
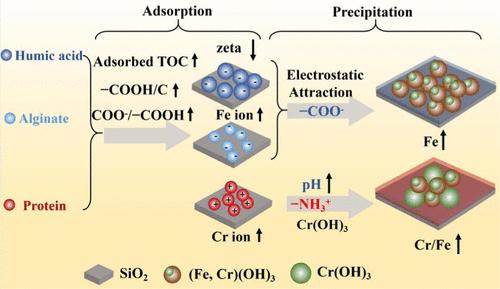
Iron/chromium hydroxide coprecipitation controls the fate and transport of toxic chromium (Cr) in many natural and engineered systems. Organic coatings on soil and engineered surfaces are ubiquitous; however, mechanistic controls of these organic coatings over Fe/Cr hydroxide coprecipitation are poorly understood. Here, Fe/Cr hydroxide coprecipitation was conducted on model organic coatings of humic acid (HA), sodium alginate (SA), and bovine serum albumin (BSA). The organics bonded with SiO2 through ligand exchange with carboxyl (–COOH), and the adsorbed amounts and pKa values of –COOH controlled surface charges of coatings. The adsorbed organic films also had different complexation capacities with Fe/Cr ions and Fe/Cr hydroxide particles, resulting in significant differences in both the amount (on HA > SA(–COOH) ≫ BSA(–NH2)) and composition (Cr/Fe molar ratio: on BSA(–NH2) ≫ HA > SA(–COOH)) of heterogeneous precipitates. Negatively charged –COOH attracted more Fe ions and oligomers of hydrolyzed Fe/Cr species and subsequently promoted heterogeneous precipitation of Fe/Cr hydroxide nanoparticles. Organic coatings containing –NH2 were positively charged at acidic pH because of the high pKa value of the functional group, limiting cation adsorption and formation of coprecipitates. Meanwhile, the higher local pH near the –NH2 coatings promoted the formation of Cr(OH)3. This study advances fundamental understanding of heterogeneous Fe/Cr hydroxide coprecipitation on organics, which is essential for successful Cr remediation and removal in both natural and engineered settings, as well as the synthesis of Cr-doped iron (oxy)hydroxides for material applications.
中文翻译:

Fe/Cr 氢氧化物在有机-水界面处的共沉淀:官能团丰富度和(去)质子化控制量和共沉淀物的组成
铁/氢氧化铬共沉淀控制着许多天然和工程系统中有毒铬 (Cr) 的命运和运输。土壤和工程表面上的有机涂层无处不在;然而,人们对这些有机涂层对 Fe/Cr 氢氧化物共沉淀的机械控制知之甚少。在这里,对腐殖酸 (HA)、海藻酸钠 (SA) 和牛血清白蛋白 (BSA) 的模型有机涂层进行 Fe/Cr 氢氧化物共沉淀。有机物通过与羧基(-COOH)的配体交换与SiO 2结合,-COOH的吸附量和p K a值控制涂层的表面电荷。吸附的有机膜与 Fe/Cr 离子和 Fe/Cr 氢氧化物颗粒也具有不同的络合能力,导致数量(HA > SA(–COOH) ≫ BSA(–NH 2 ))和组成(Cr /Fe 摩尔比:非均相沉淀的 BSA(–NH 2 ) ≫ HA > SA(–COOH))。带负电荷的-COOH吸引更多的Fe离子和水解Fe/Cr物质的低聚物,随后促进Fe/Cr氢氧化物纳米粒子的异质沉淀。由于官能团的高p K a值,含有–NH 2的有机涂层在酸性pH下带正电,限制了阳离子吸附和共沉淀物的形成。同时,–NH 2涂层附近较高的局部pH促进了Cr(OH) 3的形成。 这项研究增进了对有机物非均相 Fe/Cr 氢氧化物共沉淀的基本理解,这对于在自然和工程环境中成功修复和去除 Cr,以及合成用于材料应用的 Cr 掺杂铁(氧)氢氧化物至关重要。
更新日期:2024-05-02
中文翻译:

Fe/Cr 氢氧化物在有机-水界面处的共沉淀:官能团丰富度和(去)质子化控制量和共沉淀物的组成
铁/氢氧化铬共沉淀控制着许多天然和工程系统中有毒铬 (Cr) 的命运和运输。土壤和工程表面上的有机涂层无处不在;然而,人们对这些有机涂层对 Fe/Cr 氢氧化物共沉淀的机械控制知之甚少。在这里,对腐殖酸 (HA)、海藻酸钠 (SA) 和牛血清白蛋白 (BSA) 的模型有机涂层进行 Fe/Cr 氢氧化物共沉淀。有机物通过与羧基(-COOH)的配体交换与SiO 2结合,-COOH的吸附量和p K a值控制涂层的表面电荷。吸附的有机膜与 Fe/Cr 离子和 Fe/Cr 氢氧化物颗粒也具有不同的络合能力,导致数量(HA > SA(–COOH) ≫ BSA(–NH 2 ))和组成(Cr /Fe 摩尔比:非均相沉淀的 BSA(–NH 2 ) ≫ HA > SA(–COOH))。带负电荷的-COOH吸引更多的Fe离子和水解Fe/Cr物质的低聚物,随后促进Fe/Cr氢氧化物纳米粒子的异质沉淀。由于官能团的高p K a值,含有–NH 2的有机涂层在酸性pH下带正电,限制了阳离子吸附和共沉淀物的形成。同时,–NH 2涂层附近较高的局部pH促进了Cr(OH) 3的形成。 这项研究增进了对有机物非均相 Fe/Cr 氢氧化物共沉淀的基本理解,这对于在自然和工程环境中成功修复和去除 Cr,以及合成用于材料应用的 Cr 掺杂铁(氧)氢氧化物至关重要。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号