当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological evaluation of 5-(1H-indol-5-yl)isoxazole-3-carboxylic acids as novel xanthine oxidase inhibitors
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-04-26 , DOI: 10.1016/j.ejmech.2024.116443 Dongqian Huang 1 , Wenye Li 1 , Yilan Zhao 1 , Cheng Xie 1 , Xiaogang Luo 2 , Fengshou Wu 1 , Zhiqiang Xu 1 , Qi Sun 1 , Genyan Liu 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-04-26 , DOI: 10.1016/j.ejmech.2024.116443 Dongqian Huang 1 , Wenye Li 1 , Yilan Zhao 1 , Cheng Xie 1 , Xiaogang Luo 2 , Fengshou Wu 1 , Zhiqiang Xu 1 , Qi Sun 1 , Genyan Liu 1
Affiliation
|
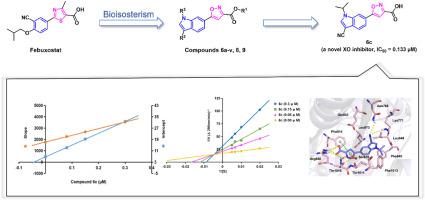
Xanthine oxidase (XO) is a key enzyme for the production of uric acid in the human body. XO inhibitors (XOIs) are clinically used for the treatment of hyperuricemia and gout, as they can effectively inhibit the production of uric acid. Previous studies indicated that both indole and isoxazole derivatives have good inhibitory effects against XO. Here, we designed and synthesized a novel series of -5-(1-indol-5-yl)isoxazole-3-carboxylic acids according to bioisosteric replacement and hybridization strategies. Among the obtained target compounds, compound showed the best inhibitory activity against XO with an IC value of 0.13 μM, which was 22-fold higher than that of the classical antigout drug allopurinol (IC = 2.93 μM). Structure-activity relationship analysis indicated that the hydrophobic group on the nitrogen atom of the indole ring is essential for the inhibitory potencies of target compounds against XO. Enzyme kinetic studies proved that compound acted as a mixed-type XOI. Molecular docking studies showed that the target compound could not only retain the key interactions similar to febuxostat at the XO binding site but also generate some new interactions, such as two hydrogen bonds between the oxygen atom of the isoxazole ring and the amino acid residues Ser876 and Thr1010. These results indicated that 5-(1-indol-5-yl)isoxazole-3-carboxylic acid might be an efficacious scaffold for designing novel XOIs and compound has the potential to be used as a lead for further the development of novel anti-gout candidates.
中文翻译:

新型黄嘌呤氧化酶抑制剂 5-(1H-吲哚-5-基)异恶唑-3-羧酸的设计、合成和生物学评价
黄嘌呤氧化酶(XO)是人体内产生尿酸的关键酶。 XO抑制剂(XOIs)在临床上用于治疗高尿酸血症和痛风,因为它们可以有效抑制尿酸的产生。前期研究表明吲哚和异恶唑衍生物均对XO具有良好的抑制作用。在这里,我们根据生物等排取代和杂交策略设计并合成了一系列新型-5-(1-吲哚-5-基)异恶唑-3-羧酸。获得的目标化合物中,该化合物对XO的抑制活性最好,IC50值为0.13μM,比经典抗痛风药物别嘌呤醇(IC50=2.93μM)高22倍。构效关系分析表明,吲哚环氮原子上的疏水基团对于目标化合物对XO的抑制效力至关重要。酶动力学研究证明该化合物为混合型XOI。分子对接研究表明,目标化合物不仅在XO结合位点保留了与非布索坦类似的关键相互作用,而且还产生了一些新的相互作用,例如异恶唑环的氧原子与氨基酸残基Ser876和Ser876之间的两个氢键Thr1010。这些结果表明5-(1-吲哚-5-基)异恶唑-3-羧酸可能是设计新型XOIs的有效支架,并且该化合物有潜力用作进一步开发新型抗痛风药物的先导物候选人。
更新日期:2024-04-26
中文翻译:

新型黄嘌呤氧化酶抑制剂 5-(1H-吲哚-5-基)异恶唑-3-羧酸的设计、合成和生物学评价
黄嘌呤氧化酶(XO)是人体内产生尿酸的关键酶。 XO抑制剂(XOIs)在临床上用于治疗高尿酸血症和痛风,因为它们可以有效抑制尿酸的产生。前期研究表明吲哚和异恶唑衍生物均对XO具有良好的抑制作用。在这里,我们根据生物等排取代和杂交策略设计并合成了一系列新型-5-(1-吲哚-5-基)异恶唑-3-羧酸。获得的目标化合物中,该化合物对XO的抑制活性最好,IC50值为0.13μM,比经典抗痛风药物别嘌呤醇(IC50=2.93μM)高22倍。构效关系分析表明,吲哚环氮原子上的疏水基团对于目标化合物对XO的抑制效力至关重要。酶动力学研究证明该化合物为混合型XOI。分子对接研究表明,目标化合物不仅在XO结合位点保留了与非布索坦类似的关键相互作用,而且还产生了一些新的相互作用,例如异恶唑环的氧原子与氨基酸残基Ser876和Ser876之间的两个氢键Thr1010。这些结果表明5-(1-吲哚-5-基)异恶唑-3-羧酸可能是设计新型XOIs的有效支架,并且该化合物有潜力用作进一步开发新型抗痛风药物的先导物候选人。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号