当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating Autoignition Characteristics of Ammonia/Heptamethylnonane Mixtures Over Wide Pressure Ranges: Rapid Compression Machine Measurements and Kinetic Modeling Study
Energy & Fuels ( IF 5.2 ) Pub Date : 2024-05-01 , DOI: 10.1021/acs.energyfuels.4c00933
Yongxiang Zhang 1 , Wei Zhou 1 , Liang Yu 1 , Xingcai Lu 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2024-05-01 , DOI: 10.1021/acs.energyfuels.4c00933
Yongxiang Zhang 1 , Wei Zhou 1 , Liang Yu 1 , Xingcai Lu 1
Affiliation
|
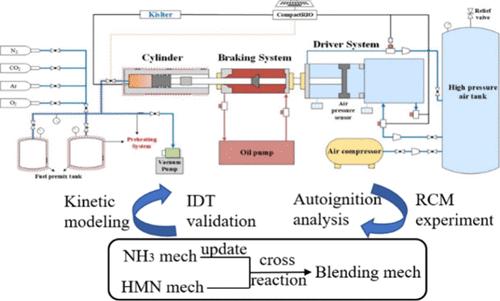
Ammonia (NH3) blending combustion with high-reactivity fuel has garnered substantial attention in terms of decarbonization potential in internal combustion engines. 2,2,4,4,6,8,8-Heptamethylnonane, denoted as HMN, an important large-molecular weight component for diesel and jet fuel surrogates, was selected to be blended with NH3 in this study. The ignition delay times (IDTs) of NH3/HMN mixtures were measured using a heated rapid compression machine (RCM) over an extensive range of conditions (temperature of 680–1025 K, pressure of 20–100 bar, equivalence ratios of 0.5–1.0, and NH3 energy ratio (NER) of 50–90%). Experimental results show that increasing the pressure, equivalence ratio, and oxygen concentration reduces both the total and first-stage IDTs, while an increase in the NH3 energy ratio prolongs the IDTs. For the mixture with the lowest NH3 energy ratio of 50%, non-Arrhenius-type behavior was observed at a pressure of 20 bar, while it transfers to a monotonic decrease of IDTs with increasing temperature at a pressure of 40 bar. An NH3/HMN blending mechanism was developed by merging individual NH3 and HMN submechanisms, updating NH3 submechanism, and adding C–N cross-reaction subset. Simulation results show that under most experimental conditions, the blending mechanism exhibits reasonable prediction on the measured NH3/HMN IDTs. Kinetic analysis shows that the discrepancy in the first-stage ignition between experiments and simulations may be associated with the inaccurate OH consumption proportion between HMN and NH3, while at the intermediate-temperature region, it may be related to the core C0–C4 mechanism and the NH3-related reactions. Further experimental or quantum calculations are needed in the future to refine the NH3/HMN blending mechanism on the basis of this work.
中文翻译:

研究宽压力范围内氨/七甲基壬烷混合物的自燃特性:快速压缩机测量和动力学建模研究
氨(NH 3)与高反应性燃料混合燃烧在内燃机脱碳潜力方面引起了广泛关注。本研究选择2,2,4,4,6,8,8-七甲基壬烷(HMN)作为柴油和喷气燃料替代品的重要大分子量成分,与NH 3混合。 NH 3 /HMN 混合物的点火延迟时间 (IDT)使用加热快速压缩机 (RCM) 在广泛的条件范围内进行测量(温度为 680–1025 K,压力为 20–100 bar,当量比为 0.5– 1.0,NH 3能量比(NER)为50-90%)。实验结果表明,增加压力、当量比和氧气浓度会降低总IDT和第一级IDT,而增加NH 3能量比会延长IDT。对于NH 3能量比最低为50%的混合物,在20 bar的压力下观察到非阿伦尼乌斯型行为,而在40 bar的压力下则转变为IDT随温度升高单调减少。通过合并单独的NH 3和HMN子机制、更新NH 3 子机制以及添加C-N交叉反应子集,开发了NH 3 /HMN混合机制。模拟结果表明,在大多数实验条件下,混合机制对测量的NH 3 /HMN IDT 表现出合理的预测。动力学分析表明,第一阶段点火实验与模拟的差异可能与HMN和NH 3之间的OH消耗比例不准确有关,而在中温区则可能与核心C 0 –C有关4机理与NH 3相关反应。未来需要进一步的实验或量子计算来在此工作的基础上完善NH 3 /HMN混合机制。
更新日期:2024-05-01
中文翻译:

研究宽压力范围内氨/七甲基壬烷混合物的自燃特性:快速压缩机测量和动力学建模研究
氨(NH 3)与高反应性燃料混合燃烧在内燃机脱碳潜力方面引起了广泛关注。本研究选择2,2,4,4,6,8,8-七甲基壬烷(HMN)作为柴油和喷气燃料替代品的重要大分子量成分,与NH 3混合。 NH 3 /HMN 混合物的点火延迟时间 (IDT)使用加热快速压缩机 (RCM) 在广泛的条件范围内进行测量(温度为 680–1025 K,压力为 20–100 bar,当量比为 0.5– 1.0,NH 3能量比(NER)为50-90%)。实验结果表明,增加压力、当量比和氧气浓度会降低总IDT和第一级IDT,而增加NH 3能量比会延长IDT。对于NH 3能量比最低为50%的混合物,在20 bar的压力下观察到非阿伦尼乌斯型行为,而在40 bar的压力下则转变为IDT随温度升高单调减少。通过合并单独的NH 3和HMN子机制、更新NH 3 子机制以及添加C-N交叉反应子集,开发了NH 3 /HMN混合机制。模拟结果表明,在大多数实验条件下,混合机制对测量的NH 3 /HMN IDT 表现出合理的预测。动力学分析表明,第一阶段点火实验与模拟的差异可能与HMN和NH 3之间的OH消耗比例不准确有关,而在中温区则可能与核心C 0 –C有关4机理与NH 3相关反应。未来需要进一步的实验或量子计算来在此工作的基础上完善NH 3 /HMN混合机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号