当前位置:
X-MOL 学术
›
Microchem. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A homogenous electrochemical biosensor for ultrasensitive ofloxacin detection based on AuNPs-enhanced synergistic amplification of enzymes
Microchemical Journal ( IF 4.9 ) Pub Date : 2024-04-27 , DOI: 10.1016/j.microc.2024.110643
Fangfang Zhou , Yue Huang , Qian Wang , Yijie Wang
Microchemical Journal ( IF 4.9 ) Pub Date : 2024-04-27 , DOI: 10.1016/j.microc.2024.110643
Fangfang Zhou , Yue Huang , Qian Wang , Yijie Wang
|
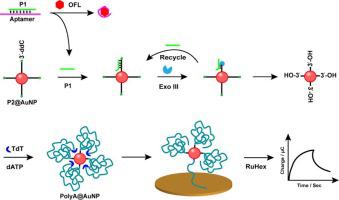
Antibiotic residue in food is still a serious health hazard to human with the various and severe adverse effects, so ultrasensitive detection of antibiotic residue becomes increasingly significant. Herein, a homogenous electrochemical biosensor is established for ultrasensitive detection of antibiotic ofloxacin (OFL) based on AuNPs-enhanced synergistic amplification of dual enzymes. Target OFL is ingeniously designed to initiate the synergistic effect of exonuclease III-catalyzed DNA cycle and terminal deoxynucleotidyl transferase-mediated polymerization, producing a great many polyA@AuNPs which subsequently adsorb onto the surface of gold electrode and finally lead to highly amplified electrochemical signal by binding with numerous electroactive indicator RuHex. Benefiting from the collaboratively multiple signal amplifications enhanced by AuNPs with good electrical conductivity and probe loading capacity, the constructed biosensor exhibits excellent quantitative detection performance for OFL with a linear range of 0.008 to 100 ng mL−1 and a limit of detection as low as 2.73 pg mL−1 . Furthermore, the detection system accomplished in homogeneous solution without complex chemical modification and rebuilding of sensing architectures on the electrode endows the biosensor with the advantages of convenient operation, good reproducibility and stability. Moreover, the biosensor shows satisfactory analytical performance for OFL detection in real food samples, providing a potential approach for practical application.
中文翻译:

一种基于 AuNPs 增强的酶协同扩增的超灵敏氧氟沙星检测的均相电化学生物传感器
食品中的抗生素残留仍然对人类健康构成严重危害,其不良影响多样且严重,因此抗生素残留的超灵敏检测变得越来越重要。在此,建立了一种均相电化学生物传感器,用于基于 AuNPs 增强的双酶协同扩增的抗生素氧氟沙星 (OFL) 的超灵敏检测。靶标 OFL 经过巧妙设计,可启动核酸外切酶 III 催化的 DNA 循环和末端脱氧核苷酸转移酶介导的聚合的协同作用,产生大量polyA@AuNPs随后吸附到金电极表面,最后通过与众多电活性指示剂 RuHex 结合导致高度放大的电化学信号。得益于具有良好导电性和探针负载能力的 AuNP 协同增强的多重信号放大,构建的生物传感器表现出优异的 OFL 定量检测性能,线性范围为 0.008 至 100 ng mL-1,检测限低至 2.73 pg mL-1。此外,在均质溶液中完成的检测系统无需复杂的化学修饰和在电极上重建传感架构,使生物传感器具有操作方便、重现性和稳定性好等优点。此外,该生物传感器对真实食品样品中 OFL 检测的分析性能令人满意,为实际应用提供了潜在的方法。
更新日期:2024-04-27
中文翻译:

一种基于 AuNPs 增强的酶协同扩增的超灵敏氧氟沙星检测的均相电化学生物传感器
食品中的抗生素残留仍然对人类健康构成严重危害,其不良影响多样且严重,因此抗生素残留的超灵敏检测变得越来越重要。在此,建立了一种均相电化学生物传感器,用于基于 AuNPs 增强的双酶协同扩增的抗生素氧氟沙星 (OFL) 的超灵敏检测。靶标 OFL 经过巧妙设计,可启动核酸外切酶 III 催化的 DNA 循环和末端脱氧核苷酸转移酶介导的聚合的协同作用,产生大量polyA@AuNPs随后吸附到金电极表面,最后通过与众多电活性指示剂 RuHex 结合导致高度放大的电化学信号。得益于具有良好导电性和探针负载能力的 AuNP 协同增强的多重信号放大,构建的生物传感器表现出优异的 OFL 定量检测性能,线性范围为 0.008 至 100 ng mL-1,检测限低至 2.73 pg mL-1。此外,在均质溶液中完成的检测系统无需复杂的化学修饰和在电极上重建传感架构,使生物传感器具有操作方便、重现性和稳定性好等优点。此外,该生物传感器对真实食品样品中 OFL 检测的分析性能令人满意,为实际应用提供了潜在的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号