当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of ricinoleic acid-based lipoamino acid derivatives
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2016-10-22 18:02:59 Y. Mohini, R.B.N. Prasad, M.S.L. Karuna, Y. Poornachandra, C. Ganesh Kumar
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2016-10-22 18:02:59 Y. Mohini, R.B.N. Prasad, M.S.L. Karuna, Y. Poornachandra, C. Ganesh Kumar
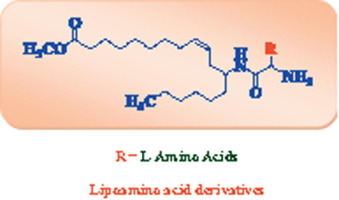
|
A series of novel ricinoleic acid based lipoamino acid derivatives were synthesized from (Z)-methyl-12-aminooctadec-9-enoate and different l-amino acids (glycine, alanine, phenyl alanine, valine, leucine, isoleucine, proline and tryptophan). The structures of all the prepared compounds were characterized by 1H NMR, 13C NMR and mass spectral studies. The title compounds were evaluated for their antimicrobial and anti-biofilm activities. Among all the derivatives, compound 7a (Z)-methyl-12-(2-aminoacetamido)octadec-9-enoate exhibited promising antibacterial activity (MIC, 3.9–7.8μg/mL) and compounds 7b (Z)-methyl 12-(2-aminopropanamido)octadec-9-enoate and 7g (Z)-methyl-12-(pyrrolidine-2-carboxamido)octadec-9-enoate exhibited moderate activity (MIC, 7.8–31.2μg/mL) selectively against four different Gram-positive bacterial strains such as Staphylococcus aureus MTCC 96, Bacillus subtilis MTCC 121, S. aureus MLS-16 MTCC 2940, Micrococcus luteus MTCC 2470. These compounds also exhibited excellent antifungal activity against studied fungal strains. Further, the compounds 7a, 7b and 7g were also screened for anti-biofilm activity. Among these lipoamino acid derivatives, compound 7a exhibited good anti-biofilm activity (IC50, 1.9–4.1μg/mL) against four Gram-positive bacterial strains.
中文翻译:

蓖麻油酸脂氨基酸衍生物的合成及生物学评价
从(Z)-甲基-12-氨基十八烷基-9-烯酸酯和不同的I-氨基酸(甘氨酸,丙氨酸,苯基丙氨酸,缬氨酸,亮氨酸,异亮氨酸,脯氨酸和色氨酸)合成了一系列基于蓖麻油酸的脂氨基酸衍生物。 。所有制备的化合物的结构均通过1 H NMR表征,1313 C NMR和质谱研究。评价标题化合物的抗微生物和抗生物膜活性。在所有衍生物中,化合物7a(Z)-甲基-12-(2-氨基乙酰胺基)octadec-9-烯酸酯显示出有希望的抗菌活性(MIC,3.9–7.8μg / mL)和化合物7b(Z)-甲基12-( 2-氨基丙酰胺基)octadec-9-烯酸酯和7g(Z)-甲基-12-(吡咯烷-2-甲酰胺基)octadec-9-烯酸酯对四种不同的革兰氏选择性药物具有中等活性(MIC,7.8-31.2μg/ mL)。阳性细菌菌株,如金黄色葡萄球菌MTCC 96,枯草芽孢杆菌MTCC 121,金黄色葡萄球菌MLS-16 MTCC 2940,黄球菌微球菌MTCC2470。这些化合物还对所研究的真菌菌株表现出出色的抗真菌活性。此外,还对化合物7a,7b和7g的抗生物膜活性进行了筛选。50,1.9-4.1μg/ mL)的对四名革兰氏阳性细菌菌株。
更新日期:2016-10-23
中文翻译:

蓖麻油酸脂氨基酸衍生物的合成及生物学评价
从(Z)-甲基-12-氨基十八烷基-9-烯酸酯和不同的I-氨基酸(甘氨酸,丙氨酸,苯基丙氨酸,缬氨酸,亮氨酸,异亮氨酸,脯氨酸和色氨酸)合成了一系列基于蓖麻油酸的脂氨基酸衍生物。 。所有制备的化合物的结构均通过1 H NMR表征,1313 C NMR和质谱研究。评价标题化合物的抗微生物和抗生物膜活性。在所有衍生物中,化合物7a(Z)-甲基-12-(2-氨基乙酰胺基)octadec-9-烯酸酯显示出有希望的抗菌活性(MIC,3.9–7.8μg / mL)和化合物7b(Z)-甲基12-( 2-氨基丙酰胺基)octadec-9-烯酸酯和7g(Z)-甲基-12-(吡咯烷-2-甲酰胺基)octadec-9-烯酸酯对四种不同的革兰氏选择性药物具有中等活性(MIC,7.8-31.2μg/ mL)。阳性细菌菌株,如金黄色葡萄球菌MTCC 96,枯草芽孢杆菌MTCC 121,金黄色葡萄球菌MLS-16 MTCC 2940,黄球菌微球菌MTCC2470。这些化合物还对所研究的真菌菌株表现出出色的抗真菌活性。此外,还对化合物7a,7b和7g的抗生物膜活性进行了筛选。50,1.9-4.1μg/ mL)的对四名革兰氏阳性细菌菌株。






























 京公网安备 11010802027423号
京公网安备 11010802027423号