当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of a 1-acyl-glycerol-3-phosphate acyltransferase from Mycobacterium tuberculosis, a key enzyme involved in triacylglycerol biosynthesis
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-04-26 , DOI: 10.1111/mmi.15265 Meghna Santoshi 1 , Harsh Bansia 1 , Muzammil Hussain 1 , Abodh Kumar Jha 1 , Valakunja Nagaraja 1, 2
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-04-26 , DOI: 10.1111/mmi.15265 Meghna Santoshi 1 , Harsh Bansia 1 , Muzammil Hussain 1 , Abodh Kumar Jha 1 , Valakunja Nagaraja 1, 2
Affiliation
|
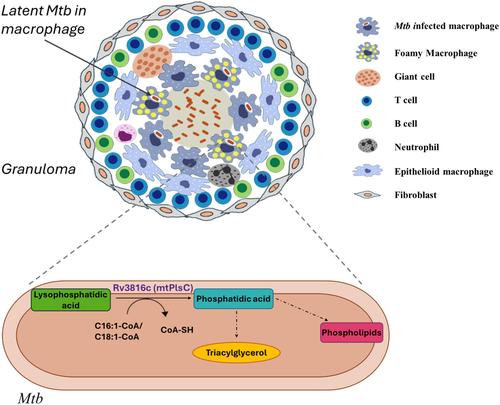
Latent tuberculosis, caused by dormant Mycobacterium tuberculosis (Mtb), poses a threat to global health through the incubation of undiagnosed infections within the community. Dormant Mtb, which is phenotypically tolerant to antibiotics, accumulates triacylglycerol (TAG) utilizing fatty acids obtained from macrophage lipid droplets. TAG is vital to mycobacteria, serving as a cell envelope component and energy reservoir during latency. TAG synthesis occurs by sequential acylation of glycerol-3-phosphate, wherein the second acylation step is catalyzed by acylglycerol-3-phosphate acyltransferase (AGPAT), resulting in the production of phosphatidic acid (PA), a precursor for the synthesis of TAG and various phospholipids. Here, we have characterized a putative acyltransferase of Mtb encoded by Rv3816c. We found that Rv3816c has all four characteristic motifs of AGPAT, exists as a membrane-bound enzyme, and functions as 1-acylglycerol-3-phosphate acyltransferase. The enzyme could transfer the acyl group to acylglycerol-3-phosphate (LPA) from monounsaturated fatty acyl-coenzyme A of chain length 16 or 18 to produce PA. Complementation of Escherichia coli PlsC mutant in vivo by Rv3816c confirmed that it functions as AGPAT. Its active site mutants, H43A and D48A, were incapable of transferring the acyl group to LPA in vitro and were not able to rescue the growth defect of E. coli PlsC mutant in vivo. Identifying Rv3816c as AGPAT and comparing its properties with other AGPAT homologs is not only a step toward understanding the TAG biosynthesis in mycobacteria but has the potential to explore it as a drug target.
中文翻译:

结核分枝杆菌中 1-酰基-甘油-3-磷酸酰基转移酶的鉴定,该酶是参与三酰基甘油生物合成的关键酶
潜伏性结核病由休眠结核分枝杆菌( Mtb ) 引起,通过在社区内潜伏未确诊的感染,对全球健康构成威胁。休眠结核分枝杆菌在表型上对抗生素具有耐受性,利用从巨噬细胞脂滴中获得的脂肪酸积累三酰甘油(TAG)。 TAG 对分枝杆菌至关重要,在潜伏期充当细胞包膜成分和能量储存库。 TAG 合成通过 3-磷酸甘油的连续酰化进行,其中第二个酰化步骤由 3-磷酸甘油酰基转移酶 (AGPAT) 催化,导致产生磷脂酸 (PA),它是 TAG 合成的前体,并且各种磷脂。在这里,我们对 Rv3816c 编码的Mtb假定酰基转移酶进行了表征。我们发现Rv3816c具有AGPAT的全部四个特征基序,作为膜结合酶存在,并作为1-酰基甘油-3-磷酸酰基转移酶发挥作用。该酶可以将链长16或18的单不饱和脂肪酰辅酶A的酰基转移到酰基甘油-3-磷酸(LPA)上以产生PA。 Rv3816c 在体内对大肠杆菌PlsC 突变体的互补证实了它具有 AGPAT 的功能。其活性位点突变体H43A和D48A无法在体外将酰基转移至LPA,并且无法在体内挽救大肠杆菌PlsC突变体的生长缺陷。将 Rv3816c 鉴定为 AGPAT 并将其特性与其他 AGPAT 同系物进行比较,不仅是了解分枝杆菌中 TAG 生物合成的一步,而且有可能将其作为药物靶点进行探索。
更新日期:2024-04-26
中文翻译:

结核分枝杆菌中 1-酰基-甘油-3-磷酸酰基转移酶的鉴定,该酶是参与三酰基甘油生物合成的关键酶
潜伏性结核病由休眠结核分枝杆菌( Mtb ) 引起,通过在社区内潜伏未确诊的感染,对全球健康构成威胁。休眠结核分枝杆菌在表型上对抗生素具有耐受性,利用从巨噬细胞脂滴中获得的脂肪酸积累三酰甘油(TAG)。 TAG 对分枝杆菌至关重要,在潜伏期充当细胞包膜成分和能量储存库。 TAG 合成通过 3-磷酸甘油的连续酰化进行,其中第二个酰化步骤由 3-磷酸甘油酰基转移酶 (AGPAT) 催化,导致产生磷脂酸 (PA),它是 TAG 合成的前体,并且各种磷脂。在这里,我们对 Rv3816c 编码的Mtb假定酰基转移酶进行了表征。我们发现Rv3816c具有AGPAT的全部四个特征基序,作为膜结合酶存在,并作为1-酰基甘油-3-磷酸酰基转移酶发挥作用。该酶可以将链长16或18的单不饱和脂肪酰辅酶A的酰基转移到酰基甘油-3-磷酸(LPA)上以产生PA。 Rv3816c 在体内对大肠杆菌PlsC 突变体的互补证实了它具有 AGPAT 的功能。其活性位点突变体H43A和D48A无法在体外将酰基转移至LPA,并且无法在体内挽救大肠杆菌PlsC突变体的生长缺陷。将 Rv3816c 鉴定为 AGPAT 并将其特性与其他 AGPAT 同系物进行比较,不仅是了解分枝杆菌中 TAG 生物合成的一步,而且有可能将其作为药物靶点进行探索。






























 京公网安备 11010802027423号
京公网安备 11010802027423号