当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DFT Insight into a Strain-Release Mechanism in Bicyclo[1.1.0]butanes via Concerted Activation of Central and Lateral C–C Bonds with Rh(III) Catalysis
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-04-27 , DOI: 10.1021/acs.inorgchem.4c00800 Jing Yan 1 , Lihua Dong 1 , Yiying Yang 2 , Dongju Zhang 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-04-27 , DOI: 10.1021/acs.inorgchem.4c00800 Jing Yan 1 , Lihua Dong 1 , Yiying Yang 2 , Dongju Zhang 2
Affiliation
|
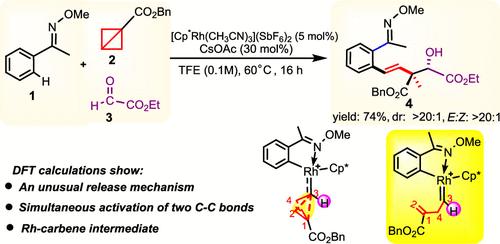
Transition-metal-catalyzed, strain-release-driven transformations of “spring-loaded” bicyclo[1.1.0]butanes (BCBs) are considered potent tools in synthetic organic chemistry. Previously proposed strain-release mechanisms involve either the insertion of the central C–C bond of BCBs into a metal–carbon bond, followed by β-C elimination, or the oxidative addition of the central or lateral C–C bond on the transition metal center, followed by reductive elimination. This study, employing DFT calculations on a Rh(III)-catalyzed model system in a three-component protocol involving oxime ether, BCB ester, and ethyl glyoxylate for constructing diastereoselective quaternary carbon centers, introduces an unusual strain-release mechanism for BCBs. In this mechanism, the catalytic reaction is initiated by the simultaneous cleavage of two C–C bonds (the central and lateral C–C bonds), resulting in the formation of a Rh-carbene intermediate. The new mechanism exhibits a barrier of 21.0 kcal/mol, making it energetically more favorable by 11.1 kcal/mol compared to the previously suggested most favorable pathway. This unusual reaction mode rationalizes experimental observation of the construction of quaternary carbon centers, including the excellent E-selectivity and diastereoselectivity. The newly proposed strain-release mechanism holds promise in advancing our understanding of transition-metal-catalyzed C–C bond activation mechanisms and facilitating the synthesis of transition metal carbene complexes.
中文翻译:

通过用 Rh(III) 催化协同激活中央和横向 C-C 键,DFT 深入了解双环[1.1.0]丁烷中的应变释放机制
过渡金属催化、应变释放驱动的“弹簧加载”双环[1.1.0]丁烷(BCB)转化被认为是有机合成化学中的有效工具。先前提出的应变释放机制涉及将 BCB 的中心 C-C 键插入金属-碳键,然后进行 β-C 消除,或者将中心或侧向 C-C 键氧化加成在过渡金属上中心,然后进行还原消除。本研究在涉及肟醚、BCB 酯和乙醛酸乙酯的三组分方案中对 Rh(III) 催化模型系统采用 DFT 计算来构建非对映选择性季碳中心,引入了 BCB 的不寻常应变释放机制。在该机制中,催化反应是由两个 C-C 键(中央和侧 C-C 键)同时断裂引发的,从而形成 Rh-卡宾中间体。新机制表现出 21.0 kcal/mol 的屏障,与之前建议的最有利途径相比,其能量更有利 11.1 kcal/mol。这种不寻常的反应模式合理化了季碳中心结构的实验观察,包括优异的E选择性和非对映选择性。新提出的应变释放机制有望增进我们对过渡金属催化的C-C键活化机制的理解,并促进过渡金属卡宾配合物的合成。
更新日期:2024-04-27
中文翻译:

通过用 Rh(III) 催化协同激活中央和横向 C-C 键,DFT 深入了解双环[1.1.0]丁烷中的应变释放机制
过渡金属催化、应变释放驱动的“弹簧加载”双环[1.1.0]丁烷(BCB)转化被认为是有机合成化学中的有效工具。先前提出的应变释放机制涉及将 BCB 的中心 C-C 键插入金属-碳键,然后进行 β-C 消除,或者将中心或侧向 C-C 键氧化加成在过渡金属上中心,然后进行还原消除。本研究在涉及肟醚、BCB 酯和乙醛酸乙酯的三组分方案中对 Rh(III) 催化模型系统采用 DFT 计算来构建非对映选择性季碳中心,引入了 BCB 的不寻常应变释放机制。在该机制中,催化反应是由两个 C-C 键(中央和侧 C-C 键)同时断裂引发的,从而形成 Rh-卡宾中间体。新机制表现出 21.0 kcal/mol 的屏障,与之前建议的最有利途径相比,其能量更有利 11.1 kcal/mol。这种不寻常的反应模式合理化了季碳中心结构的实验观察,包括优异的E选择性和非对映选择性。新提出的应变释放机制有望增进我们对过渡金属催化的C-C键活化机制的理解,并促进过渡金属卡宾配合物的合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号