当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Simulations of Interaction of the PEG-DPPE Micelle-Encapsulated Short-Chain Ceramides with the Raft-Included Membrane
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-04-23 , DOI: 10.1021/acs.jcim.4c00170 Lina Zhao 1 , Yanjiao Wang 1 , Yi Zhang 1 , Hao Chen 1 , Fude Sun 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-04-23 , DOI: 10.1021/acs.jcim.4c00170 Lina Zhao 1 , Yanjiao Wang 1 , Yi Zhang 1 , Hao Chen 1 , Fude Sun 1
Affiliation
|
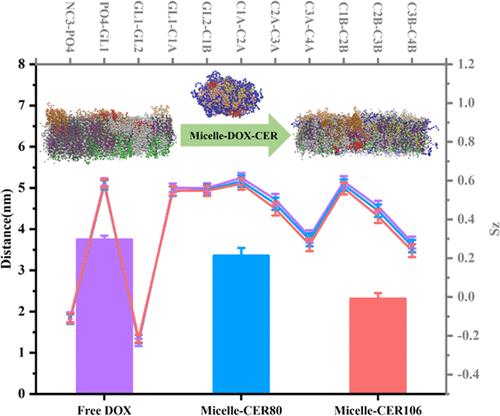
The lipid raft subdomains in cancer cell membranes play a key role in signal transduction, biomolecule recruitment, and drug transmembrane transport. Augmented membrane rigidity due to the formation of a lipid raft is unfavorable for the entry of drugs, a limiting factor in clinical oncology. The short-chain ceramide (CER) has been reported to promote drug entry into membranes and disrupt lipid raft formation, but the underlying mechanism is not well understood. We recently explored the carrier-membrane fusion dynamics of PEG-DPPE micelles in delivering doxorubicin (DOX). Based on the phase-segregated membrane model composed of DPPC/DIPC/CHOL/GM1/PIP2, we aim to explore the dynamic mechanism of the PEG-DPPE micelle-encapsulating DOXs in association with the raft-included cell membrane modulated by C8 acyl tail CERs. The results show that the lipid raft remains integrated and DOX-resistant subjected to free DOXs and the micelle-encapsulating ones. Addition of CERs disorganizes the lipid raft by pushing CHOL aside from DPPC. It subsequently allows for a good permeability for PEG-DPPE micelle-encapsulated DOXs, which penetrate deeper as CER concentration increases. GM1 is significant in guiding drugs’ redistributing between bilayer phases, and the anionic PIP2 further helps DOXs attain the inner bilayer surface. These results elaborate on the perturbing effect of CERs on lipid raft stability, which provides a new comprehensive approach for further design of drug delivery systems.
中文翻译:

PEG-DPPE胶束封装的短链神经酰胺与筏包膜相互作用的动态模拟
癌细胞膜中的脂筏亚结构域在信号转导、生物分子募集和药物跨膜转运中发挥着关键作用。由于脂筏的形成而增加的膜刚性不利于药物的进入,这是临床肿瘤学的限制因素。据报道,短链神经酰胺(CER)可以促进药物进入细胞膜并破坏脂筏的形成,但其潜在机制尚不清楚。我们最近探索了 PEG-DPPE 胶束在递送阿霉素 (DOX) 时的载体膜融合动力学。基于DPPC/DIPC/CHOL/GM1/PIP2组成的相分离膜模型,我们旨在探讨PEG-DPPE胶束封装DOX与C8酰基尾部调节的筏包含细胞膜相关的动力学机制CER。结果表明,在游离 DOX 和胶束封装的 DOX 作用下,脂筏保持完整且具有 DOX 抗性。添加 CER 会将 CHOL 推离 DPPC,从而扰乱脂筏。随后,它为 PEG-DPPE 胶束封装的 DOX 提供了良好的渗透性,随着 CER 浓度的增加,DOX 渗透得更深。 GM1 在引导药物在双层相之间重新分布方面发挥着重要作用,阴离子 PIP2 进一步帮助 DOX 到达内双层表面。这些结果详细阐述了 CER 对脂筏稳定性的扰动效应,为进一步设计药物递送系统提供了一种新的综合方法。
更新日期:2024-04-23
中文翻译:

PEG-DPPE胶束封装的短链神经酰胺与筏包膜相互作用的动态模拟
癌细胞膜中的脂筏亚结构域在信号转导、生物分子募集和药物跨膜转运中发挥着关键作用。由于脂筏的形成而增加的膜刚性不利于药物的进入,这是临床肿瘤学的限制因素。据报道,短链神经酰胺(CER)可以促进药物进入细胞膜并破坏脂筏的形成,但其潜在机制尚不清楚。我们最近探索了 PEG-DPPE 胶束在递送阿霉素 (DOX) 时的载体膜融合动力学。基于DPPC/DIPC/CHOL/GM1/PIP2组成的相分离膜模型,我们旨在探讨PEG-DPPE胶束封装DOX与C8酰基尾部调节的筏包含细胞膜相关的动力学机制CER。结果表明,在游离 DOX 和胶束封装的 DOX 作用下,脂筏保持完整且具有 DOX 抗性。添加 CER 会将 CHOL 推离 DPPC,从而扰乱脂筏。随后,它为 PEG-DPPE 胶束封装的 DOX 提供了良好的渗透性,随着 CER 浓度的增加,DOX 渗透得更深。 GM1 在引导药物在双层相之间重新分布方面发挥着重要作用,阴离子 PIP2 进一步帮助 DOX 到达内双层表面。这些结果详细阐述了 CER 对脂筏稳定性的扰动效应,为进一步设计药物递送系统提供了一种新的综合方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号