当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of digallic acid as XOD/URAT1 dual target inhibitor for the treatment of hyperuricemia
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-04-23 , DOI: 10.1016/j.bioorg.2024.107381 Fengxin Zheng 1 , Suiqing Mai 2 , Xiaolin Cen 2 , Pei Zhao 2 , Wenjie Ye 1 , Jiale Ke 1 , Shiqin Lin 2 , Huazhong Hu 2 , Zitao Guo 1 , Shuqin Zhang 2 , Hui Liao 1 , Ting Wu 1 , Yuanxin Tian 1 , Qun Zhang 2 , Jianxin Pang 3 , Zean Zhao 2
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-04-23 , DOI: 10.1016/j.bioorg.2024.107381 Fengxin Zheng 1 , Suiqing Mai 2 , Xiaolin Cen 2 , Pei Zhao 2 , Wenjie Ye 1 , Jiale Ke 1 , Shiqin Lin 2 , Huazhong Hu 2 , Zitao Guo 1 , Shuqin Zhang 2 , Hui Liao 1 , Ting Wu 1 , Yuanxin Tian 1 , Qun Zhang 2 , Jianxin Pang 3 , Zean Zhao 2
Affiliation
|
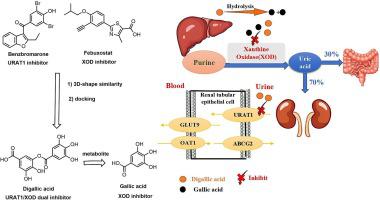
The development of XOD/URAT1 dual target inhibitors has emerged as a promising therapeutic strategy for the management of hyperuricemia. Here, through virtual screening, we have identified digallic acid as a novel dual target inhibitor of XOD/URAT1 and subsequently evaluated its pharmacological properties, pharmacokinetics, and toxicities. Digallic acid inhibited URAT1 with an IC of 5.34 ± 0.65 μM, which is less potent than benzbromarone (2.01 ± 0.36 μM) but more potent than lesinurad (10.36 ± 1.23 μM). Docking and mutation analysis indicated that residues S35, F241 and R477 of URAT1 confer a high affinity for digallic acid. Digallic acid inhibited XOD with an IC of 1.04 ± 0.23 μM. Its metabolic product, gallic acid, inhibited XOD with an IC of 0.91 ± 0.14 μM. Enzyme kinetic studies indicated that both digallic acid and gallic acid act as mixed-type XOD inhibitors. It shares the same binding mode as digallic acid, and residues E802, R880, F914, T1010, N768 and F1009 contribute to their high affinity. The anion group (carboxyl) of digallic acid contribute significantly to its inhibition activity on both XOD and URAT1 as indicated by docking analysis. Remarkably, at a dosage of 10 mg/kg digallic acid exhibited a stronger urate-lowering and uricosuric effect compared to the positive drug benzbromarone and lesinurad. Pharmacokinetic study indicated that digallic acid can be hydrolyzed into gallic acid and has a t of 0.77 ± 0.10 h. Further toxicity evaluation indicated that digallic acid exhibited no obvious renal toxicity, as reflected by CCK-8, biochemical analysis (CR and BUN) and HE examination. The findings of our study can provide valuable insights for the development of XOD/URAT1 dual target inhibitors, and digallic acid deserves further investigation as a potential anti-hyperuricemic drug.
中文翻译:

发现二没食子酸作为 XOD/URAT1 双靶点抑制剂治疗高尿酸血症
XOD/URAT1 双靶点抑制剂的开发已成为治疗高尿酸血症的一种有前途的治疗策略。在这里,通过虚拟筛选,我们确定了二没食子酸作为 XOD/URAT1 的新型双靶点抑制剂,并随后评估了其药理特性、药代动力学和毒性。二没食子酸抑制 URAT1 的 IC 值为 5.34 ± 0.65 μM,其效力低于苯溴马隆 (2.01 ± 0.36 μM),但比 lesinurad (10.36 ± 1.23 μM) 更强。对接和突变分析表明URAT1的残基S35、F241和R477赋予二没食子酸高亲和力。二没食子酸抑制 XOD,IC 为 1.04 ± 0.23 μM。其代谢产物没食子酸抑制 XOD,IC 值为 0.91 ± 0.14 μM。酶动力学研究表明二没食子酸和没食子酸都是混合型 XOD 抑制剂。它与二没食子酸具有相同的结合模式,残基 E802、R880、F914、T1010、N768 和 F1009 有助于其高亲和力。对接分析表明,二没食子酸的阴离子基团(羧基)对其对 XOD 和 URAT1 的抑制活性有显着贡献。值得注意的是,与阳性药物苯溴马隆和lesinurad相比,10mg/kg剂量的二没食子酸表现出更强的降尿酸和促尿酸排泄作用。药代动力学研究表明,二没食子酸可水解为没食子酸,水解时间为0.77±0.10 h。进一步的毒性评价表明,CCK-8、生化分析(CR和BUN)和HE检查表明,二没食子酸没有表现出明显的肾毒性。 我们的研究结果可以为XOD/URAT1双靶点抑制剂的开发提供有价值的见解,二没食子酸作为潜在的抗高尿酸血症药物值得进一步研究。
更新日期:2024-04-23
中文翻译:

发现二没食子酸作为 XOD/URAT1 双靶点抑制剂治疗高尿酸血症
XOD/URAT1 双靶点抑制剂的开发已成为治疗高尿酸血症的一种有前途的治疗策略。在这里,通过虚拟筛选,我们确定了二没食子酸作为 XOD/URAT1 的新型双靶点抑制剂,并随后评估了其药理特性、药代动力学和毒性。二没食子酸抑制 URAT1 的 IC 值为 5.34 ± 0.65 μM,其效力低于苯溴马隆 (2.01 ± 0.36 μM),但比 lesinurad (10.36 ± 1.23 μM) 更强。对接和突变分析表明URAT1的残基S35、F241和R477赋予二没食子酸高亲和力。二没食子酸抑制 XOD,IC 为 1.04 ± 0.23 μM。其代谢产物没食子酸抑制 XOD,IC 值为 0.91 ± 0.14 μM。酶动力学研究表明二没食子酸和没食子酸都是混合型 XOD 抑制剂。它与二没食子酸具有相同的结合模式,残基 E802、R880、F914、T1010、N768 和 F1009 有助于其高亲和力。对接分析表明,二没食子酸的阴离子基团(羧基)对其对 XOD 和 URAT1 的抑制活性有显着贡献。值得注意的是,与阳性药物苯溴马隆和lesinurad相比,10mg/kg剂量的二没食子酸表现出更强的降尿酸和促尿酸排泄作用。药代动力学研究表明,二没食子酸可水解为没食子酸,水解时间为0.77±0.10 h。进一步的毒性评价表明,CCK-8、生化分析(CR和BUN)和HE检查表明,二没食子酸没有表现出明显的肾毒性。 我们的研究结果可以为XOD/URAT1双靶点抑制剂的开发提供有价值的见解,二没食子酸作为潜在的抗高尿酸血症药物值得进一步研究。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号