当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of new 2-(3-(naphthalen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazole derivatives with potential analgesic and anti-inflammatory activities: In vitro, in vivo and in silico investigations
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-04-17 , DOI: 10.1016/j.bioorg.2024.107372 Eman R Mohammed 1 , Aliaa H Abd-El-Fatah 1 , Abdalla R Mohamed 2 , Marianne A Mahrouse 1 , Mohammad A Mohammad 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-04-17 , DOI: 10.1016/j.bioorg.2024.107372 Eman R Mohammed 1 , Aliaa H Abd-El-Fatah 1 , Abdalla R Mohamed 2 , Marianne A Mahrouse 1 , Mohammad A Mohammad 1
Affiliation
|
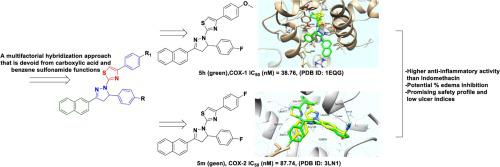
Joining the global demand for the discovery of potent NSAIDs with minimized ulcerogenic effect, new pyrazole clubbed thiazole derivatives were designed and synthesized. The new derivatives were initially evaluated for their analgesic activity. Eight compounds , , , , , , , and showed higher activity than Indomethacin (potency = 105–130 % 100 %). Subsequently, they were picked for further evaluation of their anti-inflammatory activity, ulcerogenic liability as well as toxicological studies. Derivatives and showed a potential % edema inhibition after 3 h (79.39 % and 72.12 %, respectively), with a promising safety profile and low ulcer indices (3.80 and 3.20, respectively). The two compounds and were subjected to COX-1 and COX-2 inhibition assay. The candidate showed nearly equipotent COX-1 inhibition (IC = 38.76 nM) compared to the non-selective reference drug Indomethacin (IC = 35.72 nM). Compound expressed significant inhibitory activities and a higher COX-2 selectivity index (IC = 87.74 nM, SI = 2.05) in comparison with Indomethacin (SI = 0.52), with less selectivity than Celecoxib (SI = 8.31). Simulation docking studies were carried out to gain insights into the binding interaction of compounds and in the vicinity of COX-1 and COX-2 enzymes that illustrated the importance of pyrazole clubbed thiazole core in hydrogen bonding interactions. The thiazole motif of compounds and exhibited a well orientation toward COX-1 Arg120 key residue by hydrogen bonding interactions. Compound revealed an additional arene-cation interaction with Arg120 that could rationalize its superior COX-1 inhibitory activity. Compounds and overlaid the co-crystallized ligand Celecoxib differently in the active site of COX-2. Compound showed an enhanced accommodation with binding energy of − 6.13 − 1.70 kcal/mol of compounds . The naphthalene ring of compound adopted the Celecoxib benzene sulfonamide region that is stabilized by hydrogen-arene interactions with the hydrophobic sidechains of the key residues Ser339 and Phe504. Further, the core structure of compound , pyrazole clubbed thiazole, revealed deeper hydrophobic interactions with Ala513, Leu517 and Val509 residues. Finally, a sensitive and accurate UPLC-MS/MS method was developed for the simultaneous estimation of some selected promising pyrazole derivatives in rat plasma. Accordingly, compounds and were suggested to be promising potent analgesic and anti-inflammatory agents with improved safety profiles and a novel COX isozyme modulation activity.
中文翻译:

发现具有潜在镇痛和抗炎活性的新型 2-(3-(萘-2-基)-4,5-二氢-1H-吡唑-1-基)噻唑衍生物:体外、体内和计算机研究
为了满足全球对发现具有最小化溃疡作用的强效非甾体抗炎药的需求,设计并合成了新的吡唑棒状噻唑衍生物。初步评估了新衍生物的镇痛活性。八种化合物 、 、 、 、 、 、 、 和 显示出比吲哚美辛更高的活性(效价 = 105–130 % 100 %)。随后,他们被挑选来进一步评估其抗炎活性、致溃疡能力以及毒理学研究。衍生物 和 在 3 小时后显示出潜在的水肿抑制百分比(分别为 79.39 % 和 72.12 %),具有良好的安全性和较低的溃疡指数(分别为 3.80 和 3.20)。对这两种化合物进行COX-1和COX-2抑制测定。与非选择性参考药物吲哚美辛 (IC = 35.72 nM) 相比,该候选药物表现出几乎同等的 COX-1 抑制作用 (IC = 38.76 nM)。与吲哚美辛 (SI = 0.52) 相比,该化合物表现出显着的抑制活性和更高的 COX-2 选择性指数 (IC = 87.74 nM,SI = 2.05),但选择性低于塞来昔布 (SI = 8.31)。进行了模拟对接研究,以深入了解化合物以及 COX-1 和 COX-2 酶附近的结合相互作用,说明吡唑棒状噻唑核心在氢键相互作用中的重要性。化合物的噻唑基序通过氢键相互作用表现出对 COX-1 Arg120 关键残基的良好定向。该化合物揭示了与 Arg120 的额外芳烃-阳离子相互作用,这可以合理解释其卓越的 COX-1 抑制活性。化合物和共结晶配体塞来考昔在 COX-2 的活性位点上以不同的方式重叠。化合物显示出增强的调节能力,结合能为-6。13 − 1.70 kcal/mol 化合物。化合物的萘环采用了塞来昔布苯磺酰胺区域,该区域通过与关键残基 Ser339 和 Phe504 的疏水侧链的氢-芳烃相互作用而稳定。此外,化合物的核心结构吡唑棒状噻唑揭示了与 Ala513、Leu517 和 Val509 残基更深的疏水相互作用。最后,开发了一种灵敏且准确的 UPLC-MS/MS 方法,用于同时估计大鼠血浆中一些选定的有前景的吡唑衍生物。因此,化合物和被认为是有前途的有效镇痛和抗炎剂,具有改进的安全性和新的COX同工酶调节活性。
更新日期:2024-04-17
中文翻译:

发现具有潜在镇痛和抗炎活性的新型 2-(3-(萘-2-基)-4,5-二氢-1H-吡唑-1-基)噻唑衍生物:体外、体内和计算机研究
为了满足全球对发现具有最小化溃疡作用的强效非甾体抗炎药的需求,设计并合成了新的吡唑棒状噻唑衍生物。初步评估了新衍生物的镇痛活性。八种化合物 、 、 、 、 、 、 、 和 显示出比吲哚美辛更高的活性(效价 = 105–130 % 100 %)。随后,他们被挑选来进一步评估其抗炎活性、致溃疡能力以及毒理学研究。衍生物 和 在 3 小时后显示出潜在的水肿抑制百分比(分别为 79.39 % 和 72.12 %),具有良好的安全性和较低的溃疡指数(分别为 3.80 和 3.20)。对这两种化合物进行COX-1和COX-2抑制测定。与非选择性参考药物吲哚美辛 (IC = 35.72 nM) 相比,该候选药物表现出几乎同等的 COX-1 抑制作用 (IC = 38.76 nM)。与吲哚美辛 (SI = 0.52) 相比,该化合物表现出显着的抑制活性和更高的 COX-2 选择性指数 (IC = 87.74 nM,SI = 2.05),但选择性低于塞来昔布 (SI = 8.31)。进行了模拟对接研究,以深入了解化合物以及 COX-1 和 COX-2 酶附近的结合相互作用,说明吡唑棒状噻唑核心在氢键相互作用中的重要性。化合物的噻唑基序通过氢键相互作用表现出对 COX-1 Arg120 关键残基的良好定向。该化合物揭示了与 Arg120 的额外芳烃-阳离子相互作用,这可以合理解释其卓越的 COX-1 抑制活性。化合物和共结晶配体塞来考昔在 COX-2 的活性位点上以不同的方式重叠。化合物显示出增强的调节能力,结合能为-6。13 − 1.70 kcal/mol 化合物。化合物的萘环采用了塞来昔布苯磺酰胺区域,该区域通过与关键残基 Ser339 和 Phe504 的疏水侧链的氢-芳烃相互作用而稳定。此外,化合物的核心结构吡唑棒状噻唑揭示了与 Ala513、Leu517 和 Val509 残基更深的疏水相互作用。最后,开发了一种灵敏且准确的 UPLC-MS/MS 方法,用于同时估计大鼠血浆中一些选定的有前景的吡唑衍生物。因此,化合物和被认为是有前途的有效镇痛和抗炎剂,具有改进的安全性和新的COX同工酶调节活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号