当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Study of Steering of Azido-tetrazole Behavior in Tetrazolo[1,5-c]pyrimidin-5-amine-Based Energetic Materials
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-04-25 , DOI: 10.1021/acs.joc.4c00107 Saira Manzoor 1, 2, 3 , Muhammad Adnan Younis 1, 2 , Qamar-Un-Nisa Tariq 1, 2, 3 , Jun-Qing Yang 4 , Naushad Ahmad 5 , Chuntian Qiu 1, 2 , Bingbing Tian 1 , Jian-Guo Zhang 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-04-25 , DOI: 10.1021/acs.joc.4c00107 Saira Manzoor 1, 2, 3 , Muhammad Adnan Younis 1, 2 , Qamar-Un-Nisa Tariq 1, 2, 3 , Jun-Qing Yang 4 , Naushad Ahmad 5 , Chuntian Qiu 1, 2 , Bingbing Tian 1 , Jian-Guo Zhang 3
Affiliation
|
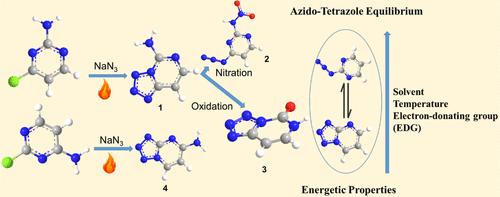
Tetrazoles and their derivatives are essential for compound synthesis due to their versatility, effectiveness, stability in air, and cost-efficiency. This has stimulated interest in developing techniques for their production. In this work, four compounds, tetrazolo[1,5-c]pyrimidin-5-amine (1), N-(4-azidopyrimidin-2-yl)nitramide (2), tetrazolo[1,5-c]pyrimidin-5(6H)-one (3), and tetrazolo[1,5-a]pyrimidin-5-amine (4), were obtained from commercially available reagents and straightforward synthetic methodologies. These new compounds were characterized by infrared (IR), 13C, and 1H NMR spectroscopy, differential scanning calorimetry (DSC), and single-crystal X-ray diffraction. The solvent, temperature, and electron-donating group (EDG) factors that were responsible for the steering of azido-tetrazole equilibrium in all compounds were also studied. In addition, the detonation performance of the target compounds was calculated by using heats of formation (HOFs) and crystal densities. Hirshfeld surface analysis was used to examine the intermolecular interactions of the four synthesized compounds. The results show that the excellent properties of 1–4 are triggered by ionic bonds, hydrogen bonds, and π–π stacking interactions, indicating that these compounds have the potential to be used in the development of high-performance energetic materials. Additionally, DFT analysis is in support of experimental results, which proved the effect of different factors that can influence the azido-tetrazole equilibrium in the synthesized pyrimidine derivatives in the solution.
中文翻译:

四唑并[1,5-c]嘧啶-5-胺基含能材料的合成及叠氮四唑行为调控研究
四唑及其衍生物由于其多功能性、有效性、空气稳定性和成本效益而对于化合物合成至关重要。这激发了开发其生产技术的兴趣。在这项工作中,四种化合物,四唑并[1,5- c ]嘧啶-5-胺( 1 )、N-(4-叠氮嘧啶-2-基)硝酰胺( 2 )、四唑并[1,5- c ]嘧啶- 5(6H)-one ( 3 ) 和四唑并[1,5- a ]嘧啶-5-胺 ( 4 ) 是通过市售试剂和简单的合成方法获得的。这些新化合物通过红外 (IR)、 13 C 和1 H NMR 光谱、差示扫描量热法 (DSC) 和单晶 X 射线衍射进行了表征。还研究了所有化合物中负责叠氮基-四唑平衡控制的溶剂、温度和给电子基团 (EDG) 因素。此外,通过使用生成热(HOF)和晶体密度计算了目标化合物的爆炸性能。赫什菲尔德表面分析用于检查四种合成化合物的分子间相互作用。结果表明, 1-4的优异性能是由离子键、氢键和π-π堆积相互作用引发的,表明这些化合物具有用于开发高性能含能材料的潜力。此外,DFT分析支持了实验结果,证明了不同因素对溶液中合成的嘧啶衍生物中叠氮基-四唑平衡的影响。
更新日期:2024-04-25
中文翻译:

四唑并[1,5-c]嘧啶-5-胺基含能材料的合成及叠氮四唑行为调控研究
四唑及其衍生物由于其多功能性、有效性、空气稳定性和成本效益而对于化合物合成至关重要。这激发了开发其生产技术的兴趣。在这项工作中,四种化合物,四唑并[1,5- c ]嘧啶-5-胺( 1 )、N-(4-叠氮嘧啶-2-基)硝酰胺( 2 )、四唑并[1,5- c ]嘧啶- 5(6H)-one ( 3 ) 和四唑并[1,5- a ]嘧啶-5-胺 ( 4 ) 是通过市售试剂和简单的合成方法获得的。这些新化合物通过红外 (IR)、 13 C 和1 H NMR 光谱、差示扫描量热法 (DSC) 和单晶 X 射线衍射进行了表征。还研究了所有化合物中负责叠氮基-四唑平衡控制的溶剂、温度和给电子基团 (EDG) 因素。此外,通过使用生成热(HOF)和晶体密度计算了目标化合物的爆炸性能。赫什菲尔德表面分析用于检查四种合成化合物的分子间相互作用。结果表明, 1-4的优异性能是由离子键、氢键和π-π堆积相互作用引发的,表明这些化合物具有用于开发高性能含能材料的潜力。此外,DFT分析支持了实验结果,证明了不同因素对溶液中合成的嘧啶衍生物中叠氮基-四唑平衡的影响。






























 京公网安备 11010802027423号
京公网安备 11010802027423号