当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Driven Decarboxylative Coupling of 2H-Indazoles with α-Keto Acids without Photocatalysts and Oxidants
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-04-20 , DOI: 10.1021/acs.joc.4c00176 Mengyu Niu 1 , Chen Yang 1 , Mingzhu Leng 1 , Qun Cao 2 , Meichao Li 1 , Zhenlu Shen 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-04-20 , DOI: 10.1021/acs.joc.4c00176 Mengyu Niu 1 , Chen Yang 1 , Mingzhu Leng 1 , Qun Cao 2 , Meichao Li 1 , Zhenlu Shen 1
Affiliation
|
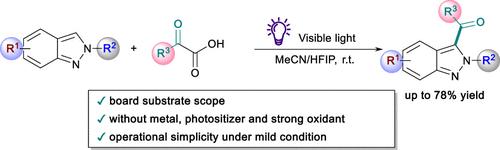
An efficient synthesis of functionalized 3-acyl-2H-indazoles via visible-light-induced self-catalyzed energy transfer was developed. This method utilized a self-catalyzed energy transfer process between 2H-indazoles and α-keto acids, offering advantages like absence of photosensitizers, metal catalysts, and strong oxidants, broad substrate compatibility, and operational simplicity under mild conditions.
中文翻译:

2H-吲唑与 α-酮酸的可见光驱动脱羧偶联,无需光催化剂和氧化剂
开发了一种通过可见光诱导自催化能量转移有效合成功能化 3-酰基-2 H-吲唑的方法。该方法利用2 H-吲唑和α-酮酸之间的自催化能量转移过程,具有无需光敏剂、金属催化剂和强氧化剂、广泛的底物兼容性以及在温和条件下操作简单等优点。
更新日期:2024-04-20
中文翻译:

2H-吲唑与 α-酮酸的可见光驱动脱羧偶联,无需光催化剂和氧化剂
开发了一种通过可见光诱导自催化能量转移有效合成功能化 3-酰基-2 H-吲唑的方法。该方法利用2 H-吲唑和α-酮酸之间的自催化能量转移过程,具有无需光敏剂、金属催化剂和强氧化剂、广泛的底物兼容性以及在温和条件下操作简单等优点。































 京公网安备 11010802027423号
京公网安备 11010802027423号