当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Azole reagents enabled ligation of peptide acyl pyrazoles for chemical protein synthesis
Chemical Science ( IF 7.6 ) Pub Date : 2024-04-25 , DOI: 10.1039/d3sc06697e
Peisi Liao 1 , Chunmao He 1
Chemical Science ( IF 7.6 ) Pub Date : 2024-04-25 , DOI: 10.1039/d3sc06697e
Peisi Liao 1 , Chunmao He 1
Affiliation
|
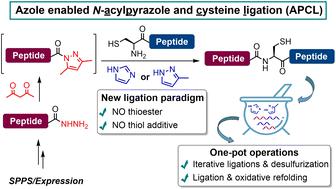
Native chemical ligation (NCL) has been playing an increasingly important role in chemical protein synthesis (CPS). More efficient ligation methods that circumvent the requirement of a peptidyl thioester and thiol additive—which allow the following desulfurization or refolding in one pot—are urgently needed for the synthesis of more complex protein targets and in large quantities. Herein, we discover that the weak acyl donor peptidyl N-acyl pyrazole can be activated by azole reagents like 3-methylpyrazole or imidazole to facilitate its ligation directly with an N-terminal cysteine peptide. As it requires no thioester or thiol additive, this ligation strategy can be conveniently combined with metal-free desulfurization (MFD) or oxidative protein folding to allow various one-pot protocols. The utility and generality of the strategy are showcased by the total synthesis of ubiquitin via an N-to-C sequential ligation–MFD strategy, the semi-synthesis of the copper protein azurin, and the efficient assembly of a sulfated hirudin variant and the cyclotide kalata B1, all in a one-pot fashion.
中文翻译:

唑试剂能够连接肽酰基吡唑以进行化学蛋白质合成
天然化学连接(NCL)在化学蛋白质合成(CPS)中发挥着越来越重要的作用。为了合成更复杂的蛋白质靶标并大量合成,迫切需要更有效的连接方法来规避肽基硫酯和硫醇添加剂的需求,从而允许在一锅中进行后续脱硫或重折叠。在此,我们发现弱酰基供体肽基N-酰基吡唑可以被唑类试剂如3-甲基吡唑或咪唑激活,以促进其与N端半胱氨酸肽直接连接。由于不需要硫酯或硫醇添加剂,这种连接策略可以方便地与无金属脱硫 (MFD) 或氧化蛋白折叠结合,以实现各种一锅法方案。通过N-to-C 顺序连接-MFD 策略全合成泛素、铜蛋白天青蛋白的半合成以及硫酸化水蛭素变体和环肽的有效组装,展示了该策略的实用性和通用性kalata B1,全部采用一锅时尚。
更新日期:2024-04-25
中文翻译:

唑试剂能够连接肽酰基吡唑以进行化学蛋白质合成
天然化学连接(NCL)在化学蛋白质合成(CPS)中发挥着越来越重要的作用。为了合成更复杂的蛋白质靶标并大量合成,迫切需要更有效的连接方法来规避肽基硫酯和硫醇添加剂的需求,从而允许在一锅中进行后续脱硫或重折叠。在此,我们发现弱酰基供体肽基N-酰基吡唑可以被唑类试剂如3-甲基吡唑或咪唑激活,以促进其与N端半胱氨酸肽直接连接。由于不需要硫酯或硫醇添加剂,这种连接策略可以方便地与无金属脱硫 (MFD) 或氧化蛋白折叠结合,以实现各种一锅法方案。通过N-to-C 顺序连接-MFD 策略全合成泛素、铜蛋白天青蛋白的半合成以及硫酸化水蛭素变体和环肽的有效组装,展示了该策略的实用性和通用性kalata B1,全部采用一锅时尚。

































 京公网安备 11010802027423号
京公网安备 11010802027423号