Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron(II) triflate as a photocatalyst for trifluoromethylation of functionalized arenes under blue LED light: Access to bioactive compounds
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-04-20 , DOI: 10.1016/j.jcat.2024.115506 Sandeep Kumawat , Kishore Natte
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-04-20 , DOI: 10.1016/j.jcat.2024.115506 Sandeep Kumawat , Kishore Natte
|
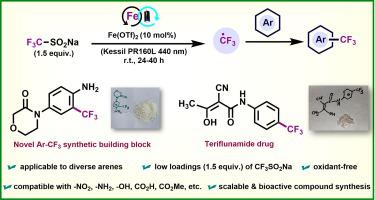
The trifluoromethyl group (-CF) containing motifs are valuable leads for the small molecule drug discovery process and have multiple applications in medicine, agriculture, and material science. Not many CF-containing motifs are commercially accessible; therefore, it is critical to design more advanced building blocks. In this work, we demonstrate an oxidant-free, scalable, and user-friendly protocol for the direct C(sp)-H trifluoromethylation of functionalized arenes using Iron(II) triflate as a robust photocatalyst and bench-stable CFSONa (1.5 equiv.) as a CF source under blue light irradiation. This method was extrapolated to >30 examples and compatible with potential functional groups (-NO, -NH, OH, -CHO, -COH, halogens, etc.). Notably, the utility of this approach is showcased for the synthesis of novel CF-containing synthetic building blocks and a few bioactive compounds.
中文翻译:

三氟甲磺酸铁 (II) 作为光催化剂,在蓝色 LED 灯下进行功能化芳烃的三氟甲基化:获取生物活性化合物
含有三氟甲基(-CF)的基序是小分子药物发现过程的宝贵先导,在医学、农业和材料科学中具有多种应用。商业上可获得的含 CF 基序并不多;因此,设计更先进的构建模块至关重要。在这项工作中,我们展示了一种无氧化剂、可扩展且用户友好的方案,使用三氟甲磺酸铁(II)作为强大的光催化剂和工作台稳定的 CFSONa(1.5 当量)对官能化芳烃进行直接 C(sp)-H 三氟甲基化。 )作为蓝光照射下的CF光源。该方法被外推到超过 30 个示例,并且与潜在的官能团(-NO、-NH、OH、-CHO、-COH、卤素等)兼容。值得注意的是,该方法在合成新型含 CF 的合成结构单元和一些生物活性化合物方面的实用性得到了展示。
更新日期:2024-04-20
中文翻译:

三氟甲磺酸铁 (II) 作为光催化剂,在蓝色 LED 灯下进行功能化芳烃的三氟甲基化:获取生物活性化合物
含有三氟甲基(-CF)的基序是小分子药物发现过程的宝贵先导,在医学、农业和材料科学中具有多种应用。商业上可获得的含 CF 基序并不多;因此,设计更先进的构建模块至关重要。在这项工作中,我们展示了一种无氧化剂、可扩展且用户友好的方案,使用三氟甲磺酸铁(II)作为强大的光催化剂和工作台稳定的 CFSONa(1.5 当量)对官能化芳烃进行直接 C(sp)-H 三氟甲基化。 )作为蓝光照射下的CF光源。该方法被外推到超过 30 个示例,并且与潜在的官能团(-NO、-NH、OH、-CHO、-COH、卤素等)兼容。值得注意的是,该方法在合成新型含 CF 的合成结构单元和一些生物活性化合物方面的实用性得到了展示。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号