当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Step and Mild Synthesis of 3-(1,3,4-Oxadiazol-2-Yl)isoindolin-1-Ones Through Ugi Four-Component Domino Dicyclization
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-04-23 , DOI: 10.1002/ajoc.202400036 Mei Sun 1 , Chong-Yang Zeng 2 , En-Shen Zhang 2 , Qiang Zhang 2 , Kai Chen 2 , Yu-Gu Xu 2 , Mei-Xia Fan 2 , Meng-Ru He 2 , Jing-Hui Shi 2 , Ming-Wu Ding 3
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-04-23 , DOI: 10.1002/ajoc.202400036 Mei Sun 1 , Chong-Yang Zeng 2 , En-Shen Zhang 2 , Qiang Zhang 2 , Kai Chen 2 , Yu-Gu Xu 2 , Mei-Xia Fan 2 , Meng-Ru He 2 , Jing-Hui Shi 2 , Ming-Wu Ding 3
Affiliation
|
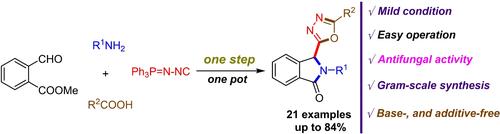
A novel and efficient Ugi four-component domino dicyclization strategy has been developed for one-step constructing diverse 3-(1,3,4-oxadiazol-2-yl)isoindolin-1-ones with good yields, without the need for additional additives. This domino dicyclization process underwent three consecutive reactions including Ugi, aza-Wittig and N-acylation by utilizing (N-isocyanimine)triphenylphosphorane, methyl 2-formylbenzoate, primary amines, and carboxylic acids. The initial biological activity testing revealed that compound 5 i exhibited good antifungal activity against certain fungi and demonstrated versatile applications in the fields of pesticide chemistry.
中文翻译:

Ugi四组分多米诺二环化一步温和合成3-(1,3,4-恶二唑-2-Yl)异吲哚啉-1-酮
开发了一种新颖高效的 Ugi 四组分多米诺二环化策略,可一步构建多种 3-(1,3,4-恶二唑-2-基)异吲哚啉-1-酮,且产率良好,无需额外添加剂。该多米诺二环化过程经历了三个连续反应,包括Ugi、氮杂维蒂希和利用(N-异氰胺)三苯基正膦、2-甲酰基苯甲酸甲酯、伯胺和羧酸的N-酰化反应。初步的生物活性测试表明,化合物5 i对某些真菌表现出良好的抗真菌活性,并在农药化学领域表现出广泛的应用。
更新日期:2024-04-23
中文翻译:

Ugi四组分多米诺二环化一步温和合成3-(1,3,4-恶二唑-2-Yl)异吲哚啉-1-酮
开发了一种新颖高效的 Ugi 四组分多米诺二环化策略,可一步构建多种 3-(1,3,4-恶二唑-2-基)异吲哚啉-1-酮,且产率良好,无需额外添加剂。该多米诺二环化过程经历了三个连续反应,包括Ugi、氮杂维蒂希和利用(N-异氰胺)三苯基正膦、2-甲酰基苯甲酸甲酯、伯胺和羧酸的N-酰化反应。初步的生物活性测试表明,化合物5 i对某些真菌表现出良好的抗真菌活性,并在农药化学领域表现出广泛的应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号