当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Mechanism of Rh(I)-Catalyzed Coupling of Benzotriazoles and Allenes Revisited: Substrate Inhibition, Proton Shuttling, and the Role of Cationic vs Neutral Species
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-04-22 , DOI: 10.1021/jacs.4c02679 Nora Jannsen 1 , Fabian Reiß 1 , Hans-Joachim Drexler 1 , Katharina Konieczny 1 , Torsten Beweries 1 , Detlef Heller 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-04-22 , DOI: 10.1021/jacs.4c02679 Nora Jannsen 1 , Fabian Reiß 1 , Hans-Joachim Drexler 1 , Katharina Konieczny 1 , Torsten Beweries 1 , Detlef Heller 1
Affiliation
|
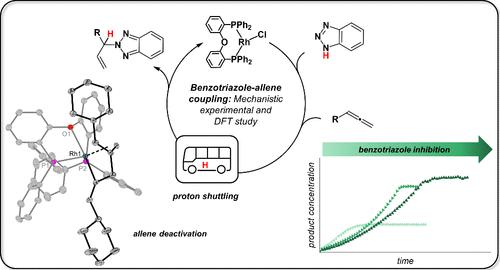
Direct coupling of benzotriazole to unsaturated substrates such as allenes represents an atom-efficient method for the construction of biologically and pharmaceutically interesting functional structures. In this work, the mechanism of the N2-selective Rh complex-catalyzed coupling of benzotriazoles to allenes was investigated in depth using a combination of experimental and theoretical techniques. Substrate coordination, inhibition, and catalyst deactivation was probed in reactions of the neutral and cationic catalyst precursors [Rh(μ-Cl)(DPEPhos)]2 and [Rh(DPEPhos)(MeOH)2]+ with benzotriazole and allene, giving coordination, or coupling of the substrates. Formation of a rhodacycle, formed by unprecedented 1,2-coupling of allenes, is responsible for catalyst deactivation. Experimental and computational data suggest that cationic species, formed either by abstraction of the chloride ligand or used directly, are relevant for catalysis. Isomerization of benzotriazole and cleavage of its N–H bond are suggested to occur by counteranion-assisted proton shuttling. This contrasts with a previously proposed scenario in which oxidative N–H addition at Rh is one of the key steps. Based on the mechanistic analysis, the catalytic coupling reaction could be optimized, leading to lower reaction temperature and shorter reaction times compared to the literature.
中文翻译:

重新审视 Rh(I) 催化苯并三唑和丙二烯的偶联机制:底物抑制、质子穿梭以及阳离子与中性物质的作用
苯并三唑与不饱和底物(例如丙二烯)的直接偶联代表了构建生物学和药学上有趣的功能结构的原子有效方法。在这项工作中,结合实验和理论技术,深入研究了N 2选择性Rh配合物催化苯并三唑与丙二烯的偶联机理。在中性和阳离子催化剂前体 [Rh(μ-Cl)(DPEPhos)] 2和 [Rh(DPEPhos)(MeOH) 2 ] +与苯并三唑和丙二烯的反应中探讨了底物配位、抑制和催化剂失活,产生配位,或基板的耦合。由丙二烯前所未有的 1,2-偶合形成的铑环的形成是催化剂失活的原因。实验和计算数据表明,通过提取氯配体形成或直接使用的阳离子物质与催化相关。苯并三唑的异构化及其 N-H 键的断裂被认为是通过抗衡阴离子辅助的质子穿梭发生的。这与之前提出的方案形成鲜明对比,其中 Rh 处的氧化 N-H 加成是关键步骤之一。基于机理分析,可以优化催化偶联反应,与文献相比,反应温度更低,反应时间更短。
更新日期:2024-04-22
中文翻译:

重新审视 Rh(I) 催化苯并三唑和丙二烯的偶联机制:底物抑制、质子穿梭以及阳离子与中性物质的作用
苯并三唑与不饱和底物(例如丙二烯)的直接偶联代表了构建生物学和药学上有趣的功能结构的原子有效方法。在这项工作中,结合实验和理论技术,深入研究了N 2选择性Rh配合物催化苯并三唑与丙二烯的偶联机理。在中性和阳离子催化剂前体 [Rh(μ-Cl)(DPEPhos)] 2和 [Rh(DPEPhos)(MeOH) 2 ] +与苯并三唑和丙二烯的反应中探讨了底物配位、抑制和催化剂失活,产生配位,或基板的耦合。由丙二烯前所未有的 1,2-偶合形成的铑环的形成是催化剂失活的原因。实验和计算数据表明,通过提取氯配体形成或直接使用的阳离子物质与催化相关。苯并三唑的异构化及其 N-H 键的断裂被认为是通过抗衡阴离子辅助的质子穿梭发生的。这与之前提出的方案形成鲜明对比,其中 Rh 处的氧化 N-H 加成是关键步骤之一。基于机理分析,可以优化催化偶联反应,与文献相比,反应温度更低,反应时间更短。















































 京公网安备 11010802027423号
京公网安备 11010802027423号