当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Prolinol Containing Inhibitors of Hypoxanthine–Guanine–Xanthine Phosphoribosyltransferase: Rational Structure-Based Drug Design
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-04-23 , DOI: 10.1021/acs.jmedchem.4c00021 Dianne T Keough 1 , Magdalena Petrová 2 , Gordon King 3 , Michal Kratochvíl 2, 4 , Radek Pohl 2 , Eva Doleželová 5 , Alena Zíková 5 , Luke W Guddat 1 , Dominik Rejman 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-04-23 , DOI: 10.1021/acs.jmedchem.4c00021 Dianne T Keough 1 , Magdalena Petrová 2 , Gordon King 3 , Michal Kratochvíl 2, 4 , Radek Pohl 2 , Eva Doleželová 5 , Alena Zíková 5 , Luke W Guddat 1 , Dominik Rejman 2
Affiliation
|
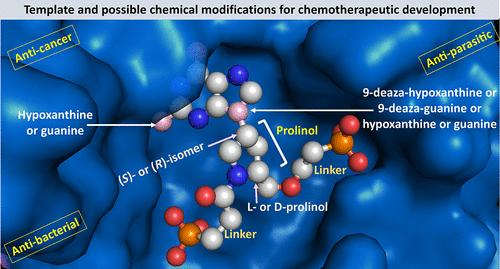
Inhibition of hypoxanthine–guanine–xanthine phosphoribosyltransferase activity decreases the pool of 6-oxo and 6-amino purine nucleoside monophosphates required for DNA and RNA synthesis, resulting in a reduction in cell growth. Therefore, inhibitors of this enzyme have potential to control infections, caused by Plasmodium falciparum and Plasmodium vivax, Trypanosoma brucei, Mycobacterium tuberculosis, and Helicobacter pylori. Five compounds synthesized here that contain a purine base covalently linked by a prolinol group to one or two phosphonate groups have Ki values ranging from 3 nM to >10 μM, depending on the structure of the inhibitor and the biological origin of the enzyme. X-ray crystal structures show that, on binding, these prolinol-containing inhibitors stimulated the movement of active site loops in the enzyme. Against TBr in cell culture, a prodrug exhibited an EC50 of 10 μM. Thus, these compounds are excellent candidates for further development as drug leads against infectious diseases as well as being potential anticancer agents.
中文翻译:

含脯氨醇的次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖基转移酶抑制剂的开发:基于合理结构的药物设计
抑制次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖转移酶活性会减少 DNA 和 RNA 合成所需的 6-氧代和 6-氨基嘌呤核苷单磷酸池,从而导致细胞生长减少。因此,这种酶的抑制剂有可能控制由恶性疟原虫和间日疟原虫、布氏锥虫、结核分枝杆菌和幽门螺杆菌引起的感染。这里合成的五种化合物含有通过脯氨醇基团与一个或两个膦酸酯基团共价连接的嘌呤碱基,其K i值范围为 3 nM 至 > 10 μM,具体取决于抑制剂的结构和酶的生物起源。 X射线晶体结构表明,在结合时,这些含脯氨醇的抑制剂刺激了酶中活性位点环的运动。针对细胞培养中的TBr ,前药的 EC 50为 10 μM。因此,这些化合物是进一步开发的优秀候选物,作为对抗传染病的先导药物以及潜在的抗癌剂。
更新日期:2024-04-23
中文翻译:

含脯氨醇的次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖基转移酶抑制剂的开发:基于合理结构的药物设计
抑制次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖转移酶活性会减少 DNA 和 RNA 合成所需的 6-氧代和 6-氨基嘌呤核苷单磷酸池,从而导致细胞生长减少。因此,这种酶的抑制剂有可能控制由恶性疟原虫和间日疟原虫、布氏锥虫、结核分枝杆菌和幽门螺杆菌引起的感染。这里合成的五种化合物含有通过脯氨醇基团与一个或两个膦酸酯基团共价连接的嘌呤碱基,其K i值范围为 3 nM 至 > 10 μM,具体取决于抑制剂的结构和酶的生物起源。 X射线晶体结构表明,在结合时,这些含脯氨醇的抑制剂刺激了酶中活性位点环的运动。针对细胞培养中的TBr ,前药的 EC 50为 10 μM。因此,这些化合物是进一步开发的优秀候选物,作为对抗传染病的先导药物以及潜在的抗癌剂。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号