Environmental Science and Pollution Research Pub Date : 2024-04-22 , DOI: 10.1007/s11356-024-33406-7 Lehlogonolo Shane Tabana , Gbolahan Joseph Adekoya , Shepherd Masimba Tichapondwa
|
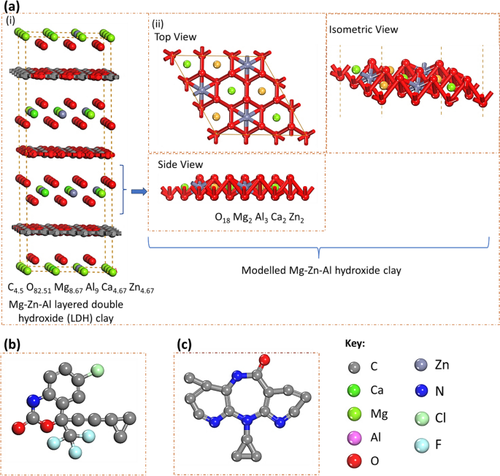
This study focused on the efficacy of a calcined layered double hydroxide (CLDH) clay in adsorbing two antiretroviral drugs (ARVDs), namely efavirenz (EFV) and nevirapine (NVP), from wastewater. The clay was synthesized using the co-precipitation method, followed by subsequent calcination in a muffle furnace at 500 °C for 4 h. The neat and calcined clay samples were subjected to various characterization techniques to elucidate their physical and chemical properties. Response surface modelling (RSM) was used to evaluate the interactions between the solution’s initial pH, adsorbent loading, reaction temperature, and initial pollutant concentration. Additionally, the adsorption kinetics, thermodynamics, and reusability of the adsorbent were evaluated. The results demonstrated that NVP exhibited a faster adsorption rate than EFV, with both reaching equilibrium within 20–24 h. The pseudo-second order (PSO) model provided a good fit for the kinetics data. Thermodynamics analysis revealed that the adsorption process was spontaneous and exothermic, predominantly governed by physisorption interactions. The adsorption isotherms followed the Freundlich model, and the maximum adsorption capacities for EFV and NVP were established to be 2.73 mg/g and 2.93 mg/g, respectively. Evaluation of the adsorption mechanism through computational analysis demonstrated that both NVP and EFV formed stable complexes with CLDH, with NVP exhibiting a higher affinity. The associated adsorption energies were established to be −731.78 kcal/mol for NVP and −512.6 kcal/mol for EFV. Visualized non-covalent interaction (NCI) graphs indicated that hydrogen bonding played a significant role in ARVDs-CLDH interactions, further emphasizing physisorption as the dominant adsorption mechanism.
中文翻译:

煅烧层状双氢氧化物粘土抗逆转录病毒药物吸附的综合研究:实验和计算分析
本研究重点研究了煅烧层状双氢氧化物 (CLDH) 粘土从废水中吸附两种抗逆转录病毒药物 (ARVD),即依非韦伦 (EFV) 和奈韦拉平 (NVP) 的功效。采用共沉淀法合成粘土,随后在马弗炉中于500℃下煅烧4小时。对纯净和煅烧的粘土样品进行了各种表征技术,以阐明其物理和化学性质。响应面模型 (RSM) 用于评估溶液初始 pH、吸附剂负载、反应温度和初始污染物浓度之间的相互作用。此外,还评估了吸附剂的吸附动力学、热力学和可重复使用性。结果表明,NVP 的吸附速率比 EFV 更快,两者均在 20-24 小时内达到平衡。伪二阶 (PSO) 模型非常适合动力学数据。热力学分析表明,吸附过程是自发的、放热的,主要受物理吸附相互作用的控制。吸附等温线遵循Freundlich模型,EFV和NVP的最大吸附容量分别为2.73 mg/g和2.93 mg/g。通过计算分析评估吸附机制表明,NVP 和 EFV 均与 CLDH 形成稳定的复合物,其中 NVP 表现出更高的亲和力。 NVP 的相关吸附能为-731.78 kcal/mol,EFV 的相关吸附能为-512.6 kcal/mol。可视化非共价相互作用 (NCI) 图表明氢键在 ARVDs-CLDH 相互作用中发挥着重要作用,进一步强调了物理吸附作为主要吸附机制。






































 京公网安备 11010802027423号
京公网安备 11010802027423号