当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring cyclopropylamine containing cyanopyrimidines as LSD1 inhibitors: Design, synthesis, ADMET, MD analysis and anticancer activity profiling
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.bioorg.2024.107336 Khursheed Ahmad Sheikh 1 , Darakhshan Parveen 1 , M Mumtaz Alam 1 , Faizul Azam 2 , Mohammad Ahmed Khan 3 , Mymoona Akhter 1 , Sharba Tasneem 1 , Meenu 1 , Suhel Parvez 4 , Khalid Imtiyaz 5 , Moshahid A Rizvi 5 , M Shaquiquzzaman 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.bioorg.2024.107336 Khursheed Ahmad Sheikh 1 , Darakhshan Parveen 1 , M Mumtaz Alam 1 , Faizul Azam 2 , Mohammad Ahmed Khan 3 , Mymoona Akhter 1 , Sharba Tasneem 1 , Meenu 1 , Suhel Parvez 4 , Khalid Imtiyaz 5 , Moshahid A Rizvi 5 , M Shaquiquzzaman 1
Affiliation
|
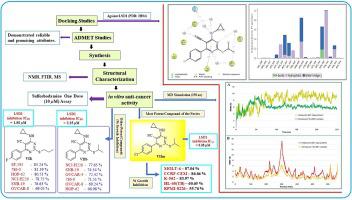
In this series we report the structure-based design, synthesis and anticancer activity evaluation of a series of eighteen cyclopropylamine containing cyanopyrimidine derivatives. The computational predictions of ADMET properties revealed appropriate aqueous solubility, high GI absorption, no BBB permeability, no Lipinski rule violations, medium total clearance and no mutagenic, tumorigenic, irritant and reproductive toxic risks for most of the compounds. Compounds and emerged as the most potent anticancer agents among all compounds evaluated against 60 cancer cell lines through the one-dose (10 µM) sulforhodamine B assay. Further, the multiple dose cell viability studies against cancer cell lines MOLT-4, A549 and HCT-116 revealed results consistent with the one-dose assay, besides sparing normal cell line HEK-293. The three potent compounds also displayed potent LSD1 inhibitory activity with IC values of 2.25, 1.80 and 6.08 µM. The -propyl-thio/isopropyl-thio group bonded to the pyrimidine ring and unsubstituted/ electron donating group (at the - position) attached to the phenyl ring resulted in enhanced anticancer activity. However, against leukemia cancer, the electron donating isopropyl group remarkably enhanced anti-cancer activity. Our findings provide important leads, which merit further optimization to result in better cancer therapeutics.
中文翻译:

探索含有氰基嘧啶的环丙胺作为 LSD1 抑制剂:设计、合成、ADMET、MD 分析和抗癌活性分析
在本系列中,我们报道了一系列十八种含环丙胺氰基嘧啶衍生物的结构设计、合成和抗癌活性评价。 ADMET 特性的计算预测显示大多数化合物具有适当的水溶性、高 GI 吸收、无 BBB 渗透性、不违反 Lipinski 规则、中等总清除率以及无致突变、致瘤、刺激和生殖毒性风险。通过单剂量 (10 µM) 磺胺罗丹明 B 测定,对 60 种癌细胞系进行了评估,这些化合物成为所有化合物中最有效的抗癌药物。此外,针对癌细胞系 MOLT-4、A549 和 HCT-116 的多剂量细胞活力研究显示,除了正常细胞系 HEK-293 之外,结果与单剂量测定一致。这三种有效的化合物还表现出有效的 LSD1 抑制活性,IC 值为 2.25、1.80 和 6.08 µM。连接至嘧啶环的-丙硫基/异丙硫基和连接至苯环的未取代/给电子基团(在-位置)导致抗癌活性增强。然而,对于白血病癌症,给电子异丙基显着增强了抗癌活性。我们的研究结果提供了重要的线索,值得进一步优化以产生更好的癌症治疗方法。
更新日期:2024-04-16
中文翻译:

探索含有氰基嘧啶的环丙胺作为 LSD1 抑制剂:设计、合成、ADMET、MD 分析和抗癌活性分析
在本系列中,我们报道了一系列十八种含环丙胺氰基嘧啶衍生物的结构设计、合成和抗癌活性评价。 ADMET 特性的计算预测显示大多数化合物具有适当的水溶性、高 GI 吸收、无 BBB 渗透性、不违反 Lipinski 规则、中等总清除率以及无致突变、致瘤、刺激和生殖毒性风险。通过单剂量 (10 µM) 磺胺罗丹明 B 测定,对 60 种癌细胞系进行了评估,这些化合物成为所有化合物中最有效的抗癌药物。此外,针对癌细胞系 MOLT-4、A549 和 HCT-116 的多剂量细胞活力研究显示,除了正常细胞系 HEK-293 之外,结果与单剂量测定一致。这三种有效的化合物还表现出有效的 LSD1 抑制活性,IC 值为 2.25、1.80 和 6.08 µM。连接至嘧啶环的-丙硫基/异丙硫基和连接至苯环的未取代/给电子基团(在-位置)导致抗癌活性增强。然而,对于白血病癌症,给电子异丙基显着增强了抗癌活性。我们的研究结果提供了重要的线索,值得进一步优化以产生更好的癌症治疗方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号