当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and characterization of indeno-fused naphthopyrans containing methacryloyl and urethane groups for photochromic contact lenses applications
European Polymer Journal ( IF 5.8 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.eurpolymj.2024.113044 Kuang-Hao Cheng , Tsung-Lin Hsieh , Shih-Jung Liu , Chi-Jui Chiang , Jyh-Chien Chen
European Polymer Journal ( IF 5.8 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.eurpolymj.2024.113044 Kuang-Hao Cheng , Tsung-Lin Hsieh , Shih-Jung Liu , Chi-Jui Chiang , Jyh-Chien Chen
|
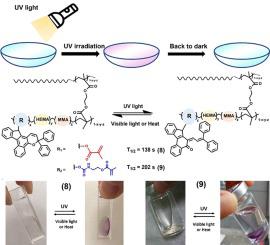
This study reports the synthesis and photochromic properties of four indeno-naphthopyrans. These compounds contain oxo, hydroxyl, methacryloyl, and urethane groups on the C13 position of indeno-fused naphthopyrans. They are light pink or colorless in solvents, and turn purple after UV irradiation. They reached the maximum optical density efficiently in 60 s in tetrahydrofuran. They also demonstrated high optical density and rapidly faded to half optical density (t) in 32–152 s. Compounds (8) (3,3-diphenyl-13-methacrylate-indeno[2,1-f]naphtho[1,2-b]pyran) and compound (9) (3,3-diphenyl-13-(2-(methacryloyloxy)ethylcarbamate)-indeno[2,1-f]naphtho[1,2-b]pyran) containing methacryloyl and urethane groups, respectively, exhibited excellent fatigue resistance after cycles of UV. Compounds (8) and (9) containing vinylic groups can polymerize with other vinyl monomers to prepare photochromic contact lenses by photo-initiated polymerization. These lenses are colorless and turn deep purple after UV irradiation. The contact lenses faded to their half optical density in 138–238 s. Although they do not fully return to their original colorless state within 3600 s after UV irradiation is turned off, the residual color is very light. They faded to 30 % of their maximum optical density in 321 to 1791 s. Additional strategies need to be explored to achieve the practical application of the photochromic contact lenses based on compounds (8) and (9).
中文翻译:

用于光致变色隐形眼镜应用的含有甲基丙烯酰基和氨基甲酸酯基团的茚并稠合萘并吡喃的合成和表征
本研究报告了四种茚并萘并吡喃的合成和光致变色特性。这些化合物在茚并稠合萘并吡喃的 C13 位上含有氧代、羟基、甲基丙烯酰基和氨基甲酸酯基团。它们在溶剂中呈浅粉色或无色,在紫外线照射后变成紫色。他们在四氢呋喃中 60 秒内有效达到最大光密度。它们还表现出高光密度,并在 32-152 秒内迅速褪色至一半光密度 (t)。化合物(8)(3,3-二苯基-13-甲基丙烯酸酯-茚并[2,1-f]萘并[1,2-b]吡喃)和化合物(9)(3,3-二苯基-13-(2-分别含有甲基丙烯酰基和氨基甲酸酯基团的(甲基丙烯酰氧基)乙基氨基甲酸酯)-茚并[2,1-f]萘并[1,2-b]吡喃)在UV循环后表现出优异的耐疲劳性。含有乙烯基的化合物(8)和(9)可以与其他乙烯基单体聚合,通过光引发聚合制备光致变色隐形眼镜。这些镜片是无色的,在紫外线照射后变成深紫色。隐形眼镜在 138-238 秒内褪色至一半光密度。尽管在关闭紫外线照射后的 3600 秒内它们不会完全恢复到原来的无色状态,但残留的颜色非常浅。它们在 321 至 1791 秒内褪至最大光密度的 30%。需要探索其他策略来实现基于化合物(8)和(9)的光致变色隐形眼镜的实际应用。
更新日期:2024-04-16
中文翻译:

用于光致变色隐形眼镜应用的含有甲基丙烯酰基和氨基甲酸酯基团的茚并稠合萘并吡喃的合成和表征
本研究报告了四种茚并萘并吡喃的合成和光致变色特性。这些化合物在茚并稠合萘并吡喃的 C13 位上含有氧代、羟基、甲基丙烯酰基和氨基甲酸酯基团。它们在溶剂中呈浅粉色或无色,在紫外线照射后变成紫色。他们在四氢呋喃中 60 秒内有效达到最大光密度。它们还表现出高光密度,并在 32-152 秒内迅速褪色至一半光密度 (t)。化合物(8)(3,3-二苯基-13-甲基丙烯酸酯-茚并[2,1-f]萘并[1,2-b]吡喃)和化合物(9)(3,3-二苯基-13-(2-分别含有甲基丙烯酰基和氨基甲酸酯基团的(甲基丙烯酰氧基)乙基氨基甲酸酯)-茚并[2,1-f]萘并[1,2-b]吡喃)在UV循环后表现出优异的耐疲劳性。含有乙烯基的化合物(8)和(9)可以与其他乙烯基单体聚合,通过光引发聚合制备光致变色隐形眼镜。这些镜片是无色的,在紫外线照射后变成深紫色。隐形眼镜在 138-238 秒内褪色至一半光密度。尽管在关闭紫外线照射后的 3600 秒内它们不会完全恢复到原来的无色状态,但残留的颜色非常浅。它们在 321 至 1791 秒内褪至最大光密度的 30%。需要探索其他策略来实现基于化合物(8)和(9)的光致变色隐形眼镜的实际应用。





























 京公网安备 11010802027423号
京公网安备 11010802027423号