当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and kinetic study of 1,3,2-dioxathiolane 2,2-dioxide in microreactors
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2024-04-17 , DOI: 10.1039/d4re00123k Ting Wang 1 , Junnan Wang 1 , Chengxiang He 1 , Zhongdong Wang 1 , Yating Li 1 , Chunying Zhu 1 , Youguang Ma 1 , Taotao Fu 1
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2024-04-17 , DOI: 10.1039/d4re00123k Ting Wang 1 , Junnan Wang 1 , Chengxiang He 1 , Zhongdong Wang 1 , Yating Li 1 , Chunying Zhu 1 , Youguang Ma 1 , Taotao Fu 1
Affiliation
|
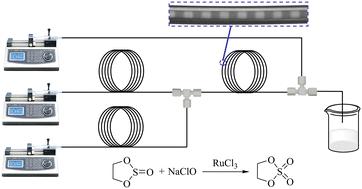
1,3,2-Dioxathiolane 2,2-dioxide (DTD) is an essential intermediate in organic synthesis and plays a vital role in improving the performance and service life of lithium-ion batteries. A large amount of heat is released during the synthesis process of DTD, leading to its hydrolysis. Therefore, the low heat exchange efficiency of traditional batch reactors leads to lower product yield and certain safety hazards. Continuous flow microreaction technology is a significant development direction for the synthesis of DTD. Based on the advantages of efficient dispersion and mixing of fluids at the micrometer scale in microreactors, the effects of temperature, catalyst concentration, residence time, ratio of the two-phase flow rates and other factors on the conversion and selectivity to DTD are investigated, and the optimal process conditions are determined. When the temperature is 14.73 °C, the catalyst concentration is 0.5 g L−1, the flow rate ratio between the continuous phase and the dispersed phase is 0.6, the total flow rate is 2 mL min−1, and the residence time is 117.75 s, the continuous reaction yield can reach 92.22%. The synthesis kinetic data of DTD are measured, and the activation energy and preexponential factor of the reaction are obtained. This study lays the foundation for the synthesis and application of DTD in microreactors, as well as the numbering-up of microreactors.
中文翻译:

微反应器中1,3,2-二氧硫杂环己烷2,2-二氧化物的合成及动力学研究
1,3,2-二氧硫杂环己烷2,2-二氧化物(DTD)是有机合成中重要的中间体,对于提高锂离子电池的性能和使用寿命起着至关重要的作用。 DTD的合成过程中释放出大量的热,导致其水解。因此,传统间歇式反应器换热效率低,导致产品收率较低,并存在一定的安全隐患。连续流微反应技术是DTD合成的一个重要发展方向。基于微反应器微米级流体高效分散和混合的优势,考察了温度、催化剂浓度、停留时间、两相流量比等因素对DTD转化率和选择性的影响,并确定了最佳工艺条件。当温度为14.73 ℃、催化剂浓度为0.5 g L -1、连续相与分散相流量比为0.6、总流量为2 mL·min -1、停留时间为117.75时s,连续反应收率可达92.22%。测量了DTD的合成动力学数据,得到了反应的活化能和指前因子。该研究为DTD在微反应器中的合成和应用以及微反应器的编号奠定了基础。
更新日期:2024-04-17
中文翻译:

微反应器中1,3,2-二氧硫杂环己烷2,2-二氧化物的合成及动力学研究
1,3,2-二氧硫杂环己烷2,2-二氧化物(DTD)是有机合成中重要的中间体,对于提高锂离子电池的性能和使用寿命起着至关重要的作用。 DTD的合成过程中释放出大量的热,导致其水解。因此,传统间歇式反应器换热效率低,导致产品收率较低,并存在一定的安全隐患。连续流微反应技术是DTD合成的一个重要发展方向。基于微反应器微米级流体高效分散和混合的优势,考察了温度、催化剂浓度、停留时间、两相流量比等因素对DTD转化率和选择性的影响,并确定了最佳工艺条件。当温度为14.73 ℃、催化剂浓度为0.5 g L -1、连续相与分散相流量比为0.6、总流量为2 mL·min -1、停留时间为117.75时s,连续反应收率可达92.22%。测量了DTD的合成动力学数据,得到了反应的活化能和指前因子。该研究为DTD在微反应器中的合成和应用以及微反应器的编号奠定了基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号