European Journal of Epidemiology ( IF 7.7 ) Pub Date : 2024-04-16 , DOI: 10.1007/s10654-024-01112-w Valentin Goutaudier 1, 2 , Marta Sablik 1 , Maud Racapé 1 , Olivia Rousseau 3, 4 , Benoit Audry 5 , Nassim Kamar 6 , Marc Raynaud 1 , Olivier Aubert 1, 2 , Béatrice Charreau 3 , Emmanuelle Papuchon 3 , Richard Danger 3 , Laurence Letertre 3 , Lionel Couzi 7 , Emmanuel Morelon 8 , Moglie Le Quintrec 9 , Jean-Luc Taupin 10 , Eric Vicaut 11 , Christophe Legendre 1, 2 , Hoa Le Mai 3 , Vishnu Potluri 12 , Thi-Van-Ha Nguyen 3 , Marie-Eliane Azoury 13 , Alice Pinheiro 13 , Georges Nouadje 13 , Pierre Sonigo 13 , Dany Anglicheau 2, 14 , Ineke Tieken 15 , Serge Vogelaar 15 , Christian Jacquelinet 5 , Peter Reese 12 , Pierre-Antoine Gourraud 3, 4 , Sophie Brouard 3 , Carmen Lefaucheur 1, 16 , Alexandre Loupy 1, 2 ,
|
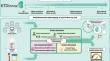
There is an unmet need for robust and clinically validated biomarkers of kidney allograft rejection. Here we present the KTD-Innov study (ClinicalTrials.gov, NCT03582436), an unselected deeply phenotyped cohort of kidney transplant recipients with a holistic approach to validate the clinical utility of precision diagnostic biomarkers. In 2018–2019, we prospectively enrolled consecutive adult patients who received a kidney allograft at seven French centers and followed them for a year. We performed multimodal phenotyping at follow-up visits, by collecting clinical, biological, immunological, and histological parameters, and analyzing a panel of 147 blood, urinary and kidney tissue biomarkers. The primary outcome was allograft rejection, assessed at each visit according to the international Banff 2019 classification. We evaluated the representativeness of participants by comparing them with patients from French, European, and American transplant programs transplanted during the same period. A total of 733 kidney transplant recipients (64.1% male and 35.9% female) were included during the study. The median follow-up after transplantation was 12.3 months (interquartile range, 11.9–13.1 months). The cumulative incidence of rejection was 9.7% at one year post-transplant. We developed a distributed and secured data repository in compliance with the general data protection regulation. We established a multimodal biomarker biobank of 16,736 samples, including 9331 blood, 4425 urinary and 2980 kidney tissue samples, managed and secured in a collaborative network involving 7 clinical centers, 4 analytical platforms and 2 industrial partners. Patients' characteristics, immune profiles and treatments closely resembled those of 41,238 French, European and American kidney transplant recipients. The KTD-Innov study is a unique holistic and multidimensional biomarker validation cohort of kidney transplant recipients representative of the real-world transplant population. Future findings from this cohort are likely to be robust and generalizable.
中文翻译:

KTD-Innov 研究的设计、队列概况和比较:肾同种异体移植排斥的前瞻性多维生物标志物验证研究
对肾同种异体移植排斥反应的可靠且经过临床验证的生物标志物的需求尚未得到满足。在这里,我们介绍 KTD-Innov 研究(ClinicalTrials.gov,NCT03582436),这是一个未经选择的肾移植受者的深度表型队列,采用整体方法来验证精确诊断生物标志物的临床效用。 2018-2019 年,我们前瞻性地连续招募了在七个法国中心接受同种异体肾脏移植的成年患者,并对他们进行了一年的随访。我们在随访时进行了多模式表型分析,收集临床、生物学、免疫学和组织学参数,并分析一组 147 种血液、尿液和肾脏组织生物标志物。主要结局是同种异体移植排斥,每次就诊时根据国际班夫 2019 年分类进行评估。我们通过将参与者与同期来自法国、欧洲和美国移植项目的患者进行比较来评估参与者的代表性。该研究共纳入了 733 名肾移植受者(64.1% 男性和 35.9% 女性)。移植后的中位随访时间为 12.3 个月(四分位数范围,11.9-13.1 个月)。移植后一年的累积排斥发生率为 9.7%。我们开发了一个符合一般数据保护法规的分布式安全数据存储库。我们建立了包含 16,736 个样本的多模式生物标志物生物库,其中包括 9331 个血液样本、4425 个尿液样本和 2980 个肾脏组织样本,并在涉及 7 个临床中心、4 个分析平台和 2 个工业合作伙伴的协作网络中进行管理和保护。 患者的特征、免疫特征和治疗与 41,238 名法国、欧洲和美国肾移植受者非常相似。 KTD-Innov 研究是一个独特的整体、多维生物标志物验证队列,对象是代表现实世界移植人群的肾移植受者。该队列的未来研究结果可能是稳健且具有普遍意义的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号