当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diels–Alder Reactivities of Strained and Unstrained Cycloalkenes with Normal and Inverse-Electron-Demand Dienes: Activation Barriers and Distortion/Interaction Analysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-10-02 , DOI: 10.1021/ja408437u Fang Liu 1 , Robert S. Paton 2 , Seonah Kim 1 , Yong Liang 1 , K. N. Houk 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-10-02 , DOI: 10.1021/ja408437u Fang Liu 1 , Robert S. Paton 2 , Seonah Kim 1 , Yong Liang 1 , K. N. Houk 1
Affiliation
|
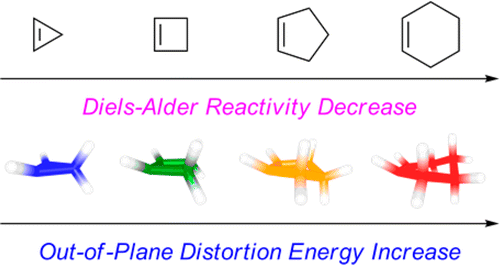
The Diels-Alder reactions of the cycloalkenes, cyclohexene through cyclopropene, with a series of dienes--1,3-dimethoxybutadiene, cyclopentadiene, 3,6-dimethyltetrazine, and 3,6-bis(trifluoromethyl)tetrazine--were studied with quantum mechanical calculations and compared with experimental values when available. The reactivities of cycloalkenes as dienophiles were found by a distortion/interaction analysis to be distortion controlled. The energies required for cycloalkenes to be distorted into the Diels-Alder transition states increase as the ring size of cycloalkenes increases from cyclopropene to cyclohexene, resulting in an increase in activation barriers. The reactivities of the dienes are controlled by both distortion and interaction energies. In normal Diels-Alder reactions with cycloalkenes, the electron-rich 1,3-dimethoxybutadiene exhibits stronger interaction energies than cyclopentadiene, but the high distortion energies required for 1,3-dimethoxybutadiene to achieve transition-state geometries overtake the favorable interaction, resulting in higher activation barriers. In inverse-electron-demand Diels-Alder reactions of 3,6-dimethyltetrazine and 3,6-bis(trifluoromethyl)tetrazine, the reactivities are mainly controlled by interaction energies.
中文翻译:

具有正常和逆电子需求二烯的应变和非应变环烯烃的 Diels-Alder 反应性:活化障碍和畸变/相互作用分析
环烯烃,环己烯通过环丙烯,与一系列二烯--1,3-二甲氧基丁二烯、环戊二烯、3,6-二甲基四嗪和3,6-双(三氟甲基)四嗪--的Diels-Alder反应进行了量子研究机械计算并与可用的实验值进行比较。通过畸变/相互作用分析发现环烯烃作为亲二烯体的反应性是畸变控制的。随着环烯烃从环丙烯到环己烯的环尺寸增加,环烯烃扭曲为 Diels-Alder 过渡态所需的能量增加,导致活化势垒增加。二烯的反应性受畸变能和相互作用能的控制。在与环烯烃的正常 Diels-Alder 反应中,富电子 1,3-二甲氧基丁二烯比环戊二烯表现出更强的相互作用能,但1,3-二甲氧基丁二烯实现过渡态几何形状所需的高畸变能超过了有利的相互作用,导致更高的活化势垒。在3,6-二甲基四嗪和3,6-双(三氟甲基)四嗪的逆电子需求Diels-Alder反应中,反应性主要受相互作用能控制。
更新日期:2013-10-02
中文翻译:

具有正常和逆电子需求二烯的应变和非应变环烯烃的 Diels-Alder 反应性:活化障碍和畸变/相互作用分析
环烯烃,环己烯通过环丙烯,与一系列二烯--1,3-二甲氧基丁二烯、环戊二烯、3,6-二甲基四嗪和3,6-双(三氟甲基)四嗪--的Diels-Alder反应进行了量子研究机械计算并与可用的实验值进行比较。通过畸变/相互作用分析发现环烯烃作为亲二烯体的反应性是畸变控制的。随着环烯烃从环丙烯到环己烯的环尺寸增加,环烯烃扭曲为 Diels-Alder 过渡态所需的能量增加,导致活化势垒增加。二烯的反应性受畸变能和相互作用能的控制。在与环烯烃的正常 Diels-Alder 反应中,富电子 1,3-二甲氧基丁二烯比环戊二烯表现出更强的相互作用能,但1,3-二甲氧基丁二烯实现过渡态几何形状所需的高畸变能超过了有利的相互作用,导致更高的活化势垒。在3,6-二甲基四嗪和3,6-双(三氟甲基)四嗪的逆电子需求Diels-Alder反应中,反应性主要受相互作用能控制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号