当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis, Relay Synthesis, and Structural Confirmation of the C18-Norditerpenoid Alkaloid Neofinaconitine
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-09-16 , DOI: 10.1021/ja4064958 Yuan Shi 1 , Jeremy T Wilmot , Lars Ulrik Nordstrøm , Derek S Tan , David Y Gin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-09-16 , DOI: 10.1021/ja4064958 Yuan Shi 1 , Jeremy T Wilmot , Lars Ulrik Nordstrøm , Derek S Tan , David Y Gin
Affiliation
|
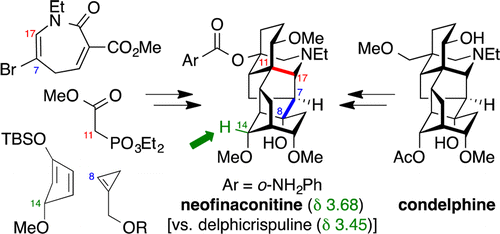
The first total synthesis of the C18-norditerpenoid aconitine alkaloid neofinaconitine and relay syntheses of neofinaconitine and 9-deoxylappaconitine from condelphine are reported. A modular, convergent synthetic approach involves initial Diels-Alder cycloaddition between two unstable components, cyclopropene 10 and cyclopentadiene 11. A second Diels-Alder reaction features the first use of an azepinone dienophile (8), with high diastereofacial selectivity achieved via rational design of siloxydiene component 36 with a sterically demanding bromine substituent. Subsequent Mannich-type N-acyliminium and radical cyclizations provide complete hexacyclic skeleton 33 of the aconitine alkaloids. Key endgame transformations include the installation of the C8-hydroxyl group via conjugate addition of water to a putative strained bridghead enone intermediate 45 and one-carbon oxidative truncation of the C4 side chain to afford racemic neofinaconitine. Complete structural confirmation was provided by a concise relay synthesis of (+)-neofinaconitine and (+)-9-deoxylappaconitine from condelphine, with X-ray crystallographic analysis of the former clarifying the NMR spectral discrepancy between neofinaconitine and delphicrispuline, which were previously assigned identical structures.
中文翻译:

C18-降二萜生物碱新非那乌丁的全合成、接力合成及结构确认
首次全合成了 C18-降二萜乌头碱生物碱新非乌头碱,并从 condelphine 中接续合成了新非乌头碱和 9-脱氧乌头碱。模块化的收敛合成方法涉及两种不稳定组分环丙烯 10 和环戊二烯 11 之间的初始 Diels-Alder 环加成。第二个 Diels-Alder 反应的特点是首次使用氮杂酮亲双烯体 (8),通过合理设计实现了高非对映选择性。具有空间要求的溴取代基的硅氧基二烯组分36。随后的曼尼希型N-酰亚胺和自由基环化提供了乌头碱生物碱的完整六环骨架33。关键的终局转化包括通过将水共轭加成到推定的应变桥头烯酮中间体 45 上来安装 C8-羟基,以及 C4 侧链的一碳氧化截短以获得外消旋新芬那乌汀。通过对来自 condelphine 的 (+)-新乌乌碱和 (+)-9-脱氧高乌乌碱进行简明中继合成,提供了完整的结构确认,并对前者进行了 X 射线晶体学分析,澄清了新乌乌乌碱和 delphicrispuline 之间的 NMR 谱差异,这是之前指定的相同的结构。
更新日期:2013-09-16
中文翻译:

C18-降二萜生物碱新非那乌丁的全合成、接力合成及结构确认
首次全合成了 C18-降二萜乌头碱生物碱新非乌头碱,并从 condelphine 中接续合成了新非乌头碱和 9-脱氧乌头碱。模块化的收敛合成方法涉及两种不稳定组分环丙烯 10 和环戊二烯 11 之间的初始 Diels-Alder 环加成。第二个 Diels-Alder 反应的特点是首次使用氮杂酮亲双烯体 (8),通过合理设计实现了高非对映选择性。具有空间要求的溴取代基的硅氧基二烯组分36。随后的曼尼希型N-酰亚胺和自由基环化提供了乌头碱生物碱的完整六环骨架33。关键的终局转化包括通过将水共轭加成到推定的应变桥头烯酮中间体 45 上来安装 C8-羟基,以及 C4 侧链的一碳氧化截短以获得外消旋新芬那乌汀。通过对来自 condelphine 的 (+)-新乌乌碱和 (+)-9-脱氧高乌乌碱进行简明中继合成,提供了完整的结构确认,并对前者进行了 X 射线晶体学分析,澄清了新乌乌乌碱和 delphicrispuline 之间的 NMR 谱差异,这是之前指定的相同的结构。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号