Chem Catalysis ( IF 11.5 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.checat.2024.100975 Baoquan Zhan , Jianxing Lv , Jiangyue Wu , Hua Zhang
|
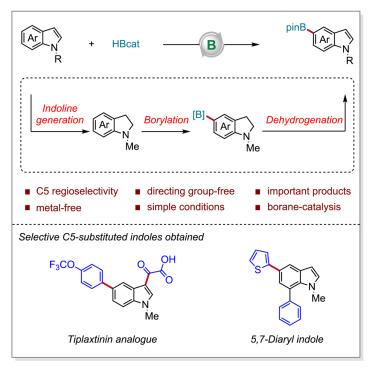
The C–H functionalization of indoles on the benzenoid moiety in preference to the more reactive pyrrolic unit is a significant challenge. Here, a borane-catalyzed C–H borylation of indoles toward the synthesis of important C5-boryl indoles has been developed. Enabled by a versatile B(C6F5)3 catalyst, various indoles reacted with HBcat to form C5-borylated indoles as well as C3, C5-diborylated indoles, which were further converted to C5-borylated indoles after protodeboronation at the C3 position under acidic conditions. Mechanistic investigations revealed a borane-catalyzed borylation/hydride transfer cascade mechanism. The synthetic potential of this transformation is also demonstrated by the concise syntheses of biorelevant C5-substituted indoles and diverse diaryl indoles.
中文翻译:

通过硼烷催化硼基化/氢化物转移级联合成 C5-硼基吲哚
苯环部分上吲哚的 C-H 官能化优先于反应性更强的吡咯单元,这是一个重大挑战。在这里,开发了一种硼烷催化的吲哚 C-H 硼基化反应,用于合成重要的 C5-硼基吲哚。在多功能B(C 6 F 5 ) 3催化剂的作用下,各种吲哚与HBcat反应形成C5-硼酸化吲哚以及C3,C5-二硼酸化吲哚,在C3位原脱硼后进一步转化为C5-硼酸化吲哚在酸性条件下。机理研究揭示了硼烷催化的硼化/氢化物转移级联机制。生物相关的 C5 取代吲哚和多种二芳基吲哚的简明合成也证明了这种转化的合成潜力。






































 京公网安备 11010802027423号
京公网安备 11010802027423号