当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-light-mediated catalyst-free synthesis of trifluoromethyl(spiro)-epoxides bearing contiguous quaternary centers
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-04-15 , DOI: 10.1039/d4qo00184b Jingchuan Lin 1 , Yu Zhang 2 , Jinxin Wang 3 , Xinyu Han 3 , Shenglan Zhu 4 , Tong Li 3 , Yanping Zhu 5 , Wei-Dong Zhang 1, 2, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-04-15 , DOI: 10.1039/d4qo00184b Jingchuan Lin 1 , Yu Zhang 2 , Jinxin Wang 3 , Xinyu Han 3 , Shenglan Zhu 4 , Tong Li 3 , Yanping Zhu 5 , Wei-Dong Zhang 1, 2, 3
Affiliation
|
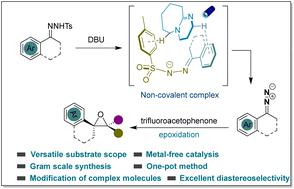
Epoxides are regarded as important pharmacophores that can have a huge effect on improving drug activity. However, epoxides with continuous quaternary centers are challenging to construct due to steric hindrance. Herein, we report a visible-light-induced cycloaddition reaction of N-tosylhydrazones with trifluoromethyl ketones to synthesize trifluoromethyl(spiro)-epoxides bearing contiguous quaternary centers under metal-free and catalyst-free conditions. This strategy could be adapted to a variety of groups and a wide range of applications, including the modification of drug derivatives and gram-scale synthesis. Furthermore, the obtained epoxides could be efficiently converted into other valuable building blocks, such as trifluoroacetone analogues.
中文翻译:

具有连续四元中心的三氟甲基(螺)环氧化物的可见光介导无催化剂合成
环氧化物被认为是重要的药效团,对提高药物活性具有巨大作用。然而,由于空间位阻,具有连续四价中心的环氧化物的构建具有挑战性。在此,我们报道了N-甲苯磺酰腙与三氟甲基酮的可见光诱导环加成反应,在无金属和无催化剂的条件下合成带有连续四元中心的三氟甲基(螺)环氧化物。该策略可以适应各种群体和广泛的应用,包括药物衍生物的修饰和克级合成。此外,获得的环氧化物可以有效地转化为其他有价值的结构单元,例如三氟丙酮类似物。
更新日期:2024-04-15
中文翻译:

具有连续四元中心的三氟甲基(螺)环氧化物的可见光介导无催化剂合成
环氧化物被认为是重要的药效团,对提高药物活性具有巨大作用。然而,由于空间位阻,具有连续四价中心的环氧化物的构建具有挑战性。在此,我们报道了N-甲苯磺酰腙与三氟甲基酮的可见光诱导环加成反应,在无金属和无催化剂的条件下合成带有连续四元中心的三氟甲基(螺)环氧化物。该策略可以适应各种群体和广泛的应用,包括药物衍生物的修饰和克级合成。此外,获得的环氧化物可以有效地转化为其他有价值的结构单元,例如三氟丙酮类似物。































 京公网安备 11010802027423号
京公网安备 11010802027423号