当前位置:
X-MOL 学术
›
J. Supercrit. Fluids
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement and prediction of CO2 solubility in organic electrolytes for high pressure CO2 reduction
The Journal of Supercritical Fluids ( IF 3.4 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.supflu.2024.106268 Marvin Dorn , Lukas Franke , Paul Figiel , Sabine Kareth , Eckhard Weidner , Christoph Held , Marcus Petermann
The Journal of Supercritical Fluids ( IF 3.4 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.supflu.2024.106268 Marvin Dorn , Lukas Franke , Paul Figiel , Sabine Kareth , Eckhard Weidner , Christoph Held , Marcus Petermann
|
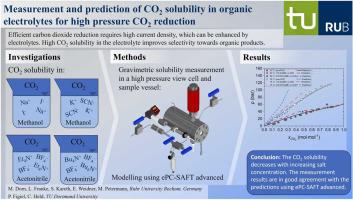
The electrochemical CO reduction reaction (CO2RR) to organic products is becoming increasingly important. Here, organic electrolytes play a crucial role, since adding salt to organic solvent allows an increase in the current density, and together with supercritical CO organic electrolytes suppress hydrogen evolution during the CO2RR. Since the selectivity to organic products depends on the CO content, CO solubility was investigated in organic electrolytes at different salt concentrations, pressures (up to 150 bar), and temperatures (25 °C and 70 °C). These organic electrolytes included the mixtures NaI-methanol, KSCN-methanol, (CH)NBF-acetonitrile, and (CH)NBF-acetonitrile. Afterwards, electrolyte Perturbed-Chain Statistical Associating Fluid Theory (ePC-SAFT) was applied to predict the CO solubility in these organic electrolytes. The results showed that i) CO solubility decreased with increasing temperature and salt concentration, ii) dissolving CO may cause a precipitation of salts, and that iii) ePC-SAFT allowed to qualitatively model the CO solubility in organic electrolytes
中文翻译:

高压 CO2 还原的有机电解质中 CO2 溶解度的测量和预测
有机产品的电化学 CO 还原反应 (CO2RR) 变得越来越重要。在这里,有机电解质起着至关重要的作用,因为在有机溶剂中添加盐可以增加电流密度,并且与超临界CO有机电解质一起抑制CO2RR过程中的析氢。由于有机产物的选择性取决于 CO 含量,因此研究了不同盐浓度、压力(高达 150 bar)和温度(25 °C 和 70 °C)下有机电解质中 CO 的溶解度。这些有机电解质包括NaI-甲醇、KSCN-甲醇、(CH)NBF-乙腈和(CH)NBF-乙腈的混合物。随后,应用电解质扰动链统计缔合流体理论(ePC-SAFT)来预测这些有机电解质中CO的溶解度。结果表明,i) CO 溶解度随着温度和盐浓度的增加而降低,ii) 溶解 CO 可能会导致盐沉淀,以及 iii) ePC-SAFT 可以定性模拟 CO 在有机电解质中的溶解度
更新日期:2024-04-06
中文翻译:

高压 CO2 还原的有机电解质中 CO2 溶解度的测量和预测
有机产品的电化学 CO 还原反应 (CO2RR) 变得越来越重要。在这里,有机电解质起着至关重要的作用,因为在有机溶剂中添加盐可以增加电流密度,并且与超临界CO有机电解质一起抑制CO2RR过程中的析氢。由于有机产物的选择性取决于 CO 含量,因此研究了不同盐浓度、压力(高达 150 bar)和温度(25 °C 和 70 °C)下有机电解质中 CO 的溶解度。这些有机电解质包括NaI-甲醇、KSCN-甲醇、(CH)NBF-乙腈和(CH)NBF-乙腈的混合物。随后,应用电解质扰动链统计缔合流体理论(ePC-SAFT)来预测这些有机电解质中CO的溶解度。结果表明,i) CO 溶解度随着温度和盐浓度的增加而降低,ii) 溶解 CO 可能会导致盐沉淀,以及 iii) ePC-SAFT 可以定性模拟 CO 在有机电解质中的溶解度
















































 京公网安备 11010802027423号
京公网安备 11010802027423号