当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Tricyclic Fused 6–5–4 Carbocycles via Acid-Promoted Cascade Intramolecular Cyclization of Allenylsilane-Tethered Cyclohexadienones
Organic Letters ( IF 4.9 ) Pub Date : 2024-04-12 , DOI: 10.1021/acs.orglett.4c01108
Yuka Iwakiri 1 , Ryoma Kishimoto 1 , Kazuhiko Sakaguchi 1 , Takahiro Nishimura 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-04-12 , DOI: 10.1021/acs.orglett.4c01108
Yuka Iwakiri 1 , Ryoma Kishimoto 1 , Kazuhiko Sakaguchi 1 , Takahiro Nishimura 1
Affiliation
|
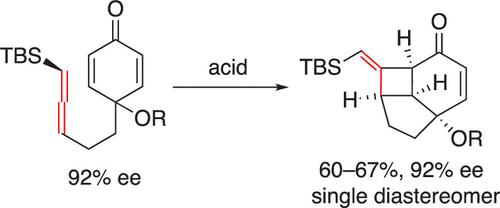
The reaction of 2,5-cyclohexadienones with methylene-tethered allenylsilane in the presence of Lewis or Brønsted acids leads to a cascade of intramolecular cyclization, yielding stereoselective tricyclic fused 6–5–4 carbocycles featuring a silyl-methylenecyclobutane ring. This transformation is notable for the diastereoselective asymmetric desymmetrization of prochiral dienones, attributed to the axial chirality of allene.
中文翻译:

通过烯基硅烷系环己二烯酮的酸促进级联分子内环化合成三环稠合 6-5-4 碳环
2,5-环己二酮与亚甲基系联烯基硅烷在路易斯酸或布朗斯台德酸存在下发生反应,导致分子内环化级联,产生具有甲硅烷基-亚甲基环丁烷环的立体选择性三环稠合6-5-4碳环。这种转变因前手性二烯酮的非对映选择性不对称去对称化而值得注意,这归因于丙二烯的轴向手性。
更新日期:2024-04-12
中文翻译:

通过烯基硅烷系环己二烯酮的酸促进级联分子内环化合成三环稠合 6-5-4 碳环
2,5-环己二酮与亚甲基系联烯基硅烷在路易斯酸或布朗斯台德酸存在下发生反应,导致分子内环化级联,产生具有甲硅烷基-亚甲基环丁烷环的立体选择性三环稠合6-5-4碳环。这种转变因前手性二烯酮的非对映选择性不对称去对称化而值得注意,这归因于丙二烯的轴向手性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号